
ORIGINAL ARTICLES
Anterior chamber drainage with a silicone tube as an adjunctive treatment for refractory endophthalmitis: a case series
Arturo Maldonado Bas, Keshet Lypnik, Betiana Caminos, Daniela Bianco
Clínica de Ojos Maldonado Bas, Córdoba City (Córdoba), Argentina.
Received: June 6th, 2025.
Approved: August 7th, 2025.
Corresponsal author
Dra. Keshet Lypnik
Clínica de Ojos Maldonado Bas
Achaval Rodríguez 544
Barrio Güemes
Córdoba (provincia de Córdoba)
Argentina
+54 (0351) 468-6500
lypnikkeshet@gmail.com
Oftalmol Clin Exp (ISSNe 1851-2658)
2025; 18(3): e328-e336.
DOI: https://doi.org/10.70313/2718.7446.v18.n3.443
Abstract
Objective: To describe a novel surgical approach using a silicone tube device for anterior chamber drainage as an adjunctive treatment in patients with refractory endophthalmitis.
Methods: We conducted a retrospective, single-center, non-randomized case series including patients with endophthalmitis of different etiologies, managed at the Maldonado Bas Eye Clinic between 2012 and 2021. All patients had received conventional treatment for endophthalmitis (topical fortified antibiotics, systemic therapy, anterior chamber lavage, vitrectomy, conjunctival flap, or keratoplasty) without satisfactory response. The technique involved the implantation of a silicone tube connecting the anterior chamber to the suprascleral space, covered by a scleral or corneoscleral patch and conjunctiva. Outcomes were assessed through infection control, best-corrected visual acuity (BCVA), and follow-up examinations at 12 months.
Results: Six patients were included. All presented with corneal abscess and signs of endophthalmitis at baseline. Five of the six patients (83%) achieved infection control after tube implantation, with no need for continued antibiotic therapy or further evidence of infection during follow-up. Visual outcomes varied according to baseline vision and severity, with final BCVA ranging from light perception to 20/25. One patient required evisceration due to uncontrolled infection.
Conclusion: Anterior chamber drainage with a silicone tube device proved to be a safe and potentially effective adjunctive technique for refractory endophthalmitis, achieving infection control in most cases. This approach should be considered as a complementary therapeutic tool within the current armamentarium against endophthalmitis, particularly in extreme scenarios where conventional management has failed. Further studies are needed to validate these findings.
Keywords: endophthalmitis, anterior chamber drainage, silicone tuve, refractory infection, ocular surgery.
Drenaje de cámara anterior con tubo de silicona como tratamiento complementario en endoftalmitis refractarias: serie de casos
Resumen
Objetivo: Describir una nueva técnica quirúrgica que utiliza un dispositivo de tubo de silicona para el drenaje de cámara anterior como tratamiento complementario en pacientes con endoftalmitis refractaria.
Métodos: Se realizó un estudio retrospectivo, unicéntrico y no randomizado, que incluyó pacientes con endoftalmitis de diferentes etiologías atendidos en la Clínica de Ojos Maldonado Bas entre 2012 y 2021. Todos los pacientes habían recibido tratamiento convencional para la endoftalmitis (colirios antibióticos fortificados, terapia sistémica, lavados de cámara anterior, vitrectomía, colgajo conjuntival o queratoplastia) sin respuesta satisfactoria. La técnica consistió en la implantación de un tubo de silicona que conecta la cámara anterior con el espacio supraescleral, cubierto por un parche escleral o córneo-escleral y conjuntiva. Los resultados se evaluaron en función del control de la infección, la agudeza visual mejor corregida (AVMC) y los controles evolutivos hasta los 12 meses.
Resultados: Se incluyeron seis pacientes. Todos presentaban absceso corneal y signos de endoftalmitis al inicio. Cinco de los seis (83%) lograron control de la infección tras la colocación del tubo sin necesidad de medicación antibiótica continua ni recurrencia infecciosa durante el seguimiento. Los resultados visuales variaron según la visión inicial y la gravedad, con AVMC final entre percepción luminosa y 20/25. Un paciente requirió evisceración por infección no controlada.
Conclusión: El drenaje de cámara anterior mediante un tubo de silicona demostró ser una técnica segura y potencialmente eficaz como tratamiento complementario en endoftalmitis refractarias, logrando control de la infección en la mayoría de los casos. Este procedimiento debe considerarse una herramienta terapéutica adicional dentro del arsenal actual contra la endoftalmitis, especialmente en escenarios extremos donde el manejo convencional resulta insuficiente. Se requieren futuros estudios para validar estos hallazgos.
Palabras clave: endoftalmitis, drenaje de cámara anterior, tubo de silicona, infección refractaria, cirugía ocular.
Drenagem de câmara anterior com tubo de silicone como tratamento complementar em endoftalmite refratária: série de casos
Resumo
Objetivo: Descrever uma nova técnica cirúrgica que utiliza um dispositivo de tubo de silicone para drenagem da câmara anterior como tratamento adjuvante em pacientes com endoftalmite refratária.
Métodos: Foi realizado um estudo retrospectivo, unicêntrico e não randomizado, que incluiu pacientes com endoftalmite de diferentes etiologias tratados na Clínica Oftalmológica Maldonado Bas entre 2012 e 2021. Todos os pacientes receberam tratamento convencional para endoftalmite (colírio antibiótico fortificado, terapia sistêmica, lavagem da câmara anterior, vitrectomia, retalho conjuntival ou ceratoplastia) sem resposta satisfatória. A técnica consistiu no implante de um tubo de silicone conectando a câmara anterior com o espaço supraescleral, coberto por um patch escleral ou córneo-escleral e conjuntival. Os resultados foram avaliados com base no controle da infecção, melhor acuidade visual corrigida (MAVC) e visitas de acompanhamento por até 12 meses.
Resultados: Seis pacientes foram incluídos. Todos apresentavam abscessos corneanos e sinais de endoftalmite na apresentação. Cinco dos seis pacientes (83%) obtiveram controle da infecção após a colocação do tubo, sem necessidade de antibioticoterapia contínua ou recorrência da infecção durante o acompanhamento. Os resultados visuais variaram de acordo com a visão basal e a gravidade, com a acuidade visual corrigida final variando de percepção luminosa a 20/25. Um paciente necessitou de evisceração devido à infecção descontrolada.
Conclusão: A drenagem da câmara anterior com tubo de silicone demonstrou ser um tratamento adjuvante seguro e potencialmente eficaz para endoftalmite refratária, alcançando o controle da infecção na maioria dos casos. Este procedimento deve ser considerado uma ferramenta terapêutica adicional dentro do arsenal terapêutico atual para endoftalmite, especialmente em casos extremos em que o tratamento convencional é insuficiente. Estudos futuros são necessários para validar esses achados.
Palavras-chave: endoftalmite, drenagem da câmara anterior, tubo de silicone, infecção refratária, cirurgia ocular.
Introduction
Endophthalmitis remains a serious challenge in ophthalmology, as it may not only result in irreversible vision loss, but can also lead to loss of the eyeball and, in rare cases, even threaten the patient’s life if the infection spreads systemically1-2. In general medicine and across several specialties, including neurology, a widely accepted adjunctive measure for managing infectious abscesses is drainage, given that antimicrobial drugs have limited penetration into the abscess cavity3-7.
Our group has longstanding experience in the study and development of aqueous humor drainage systems8, and has even proposed the potential usefulness of an oculoperitoneal shunt9. For refractory cases of endophthalmitis, our group has hypothesized that, in extreme situations, the complementary use of a drainage system could provide significant benefit in improving disease resolution. Within this framework, the present study describes a novel procedure proposed as an adjunct to the conventional management of endophthalmitis.
Materials and methods
Study design and ethics
A retrospective, single-center, non-randomized study was conducted to analyze and describe the characteristics of a case series of endophthalmitis managed at the Maldonado Bas Eye Clinic between 2012 and 2021. Patients in whom this technique was applied provided written informed consent. Likewise, participating physicians adhered to the principles outlined in the Declaration of Helsinki. The study protocol was approved by the Teaching and Research Committee of the center as a retrospective analysis.
Population
The population offered this technique consisted of patients with endophthalmitis of different etiologies, of any age (for minors, parental or legal guardian authorization was required), who had already received standardized treatment for endophthalmitis but showed poor clinical evolution or refractoriness to therapy. This included failure to topical or injectable antibiotics, anterior chamber irrigation, and/or vitrectomy. Exclusion criteria were patients with terminal illnesses such as cancer, a history of systemic pathologies that could interfere with the evaluation of the study or compromise patient safety, as well as cases presenting signs and symptoms indicating the need for evisceration.
Clinical outcomes and data analysis
The following variables were analyzed: best-corrected visual acuity (BCVA), slit-lamp examination findings including corneal characteristics (presence of corneal abscess or edema), hypopyon, Tyndall effect, and anterior or posterior synechiae. Treatment success was defined as the absence of clinical signs of endophthalmitis (Tyndall effect, hypopyon, or corneal abscess), with strict follow-up monitoring over the subsequent months. Data used for variable comparison were collected at the 12-month postoperative visit. Statistical analyses were performed using R-Medic software. Hypothesis tests for proportions and frequency distributions were applied, with results reported as 95% confidence intervals.
Surgical procedure
a. Summary: a silicone tube implant was positioned to connect the anterior chamber and suprascleral space (Fig. 1), with the distal end covered by either a scleral patch or corneoscleral ring, both placed beneath a conjunctival pocket (Fig. 2). This intrascleral tube has also been utilized for managing refractory glaucoma in patients who required filtering surgery but could not undergo the procedure or had unsuccessful outcomes from it. This study proposes employing the tube for draining the anterior chamber to address infection-related issues.

Figure 1. Silicone tube device prior to implantation. The tube is shown before surgical placement for anterior chamber drainage in refractory endophthalmitis.
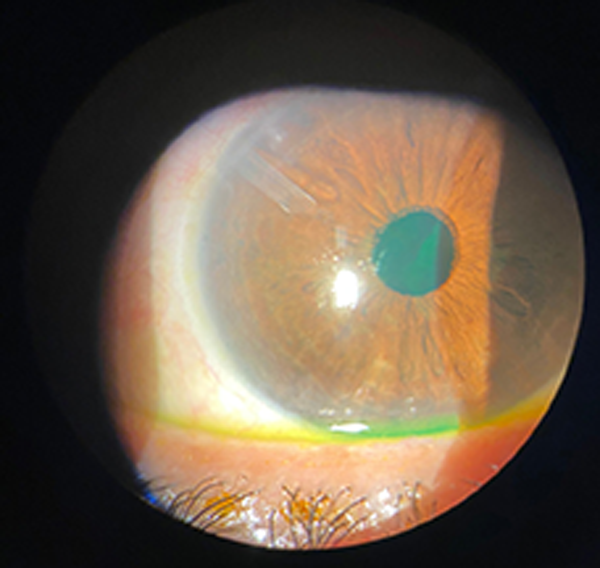
Figure 2. Silicone tube in situ. The distal end of the tube can be observed within the anterior chamber following implantation for aqueous drainage in refractory endophthalmitis.
b. Features of the microdevice: a silicone tube 10 mm long, external diameter 0.5 mm and internal diameter 0.3 mm.
c. Surgical steps:
1. Paracentesis.
2. Conjunctival incision at the base of the limbus and divulsion, creating a conjunctival pocket.
3. Hemostasis with diathermy if necessary.
4. Scleral incision with V-lance, creating a tunnel toward the anterior chamber for the tube to be inserted.
5. Lavage of the anterior chamber.
6. Insertion of the silicone tube for drainage through the tunnel created at point 4, fixing it with 10/0 prolene.
7. Check the position and permeability of the silicone tube.
8. Placement of a scleral patch over the tube, leaving the distal end of the tube free.
9. Scleral closure with 10/0 nylon sutures.
10. Conjunctival closure with 8/0 silk stitches.
11. Injection of viscoelastic into the anterior chamber.
d. Postoperative medication consisted in all cases of broad-spectrum antibiotics, covering gram-negative and positive bacteria. Depending on the case, medication directed at the etiologic agent was added and progressively withdrawn during subsequent check-ups as the patient showed signs of improvement.
General characteristics of the case series
At the 12-month follow-up after device implantation, five of the six patients were considered cured, defined as the absence of infection signs including corneal abscess, Tyndall effect, and hypopyon. During subsequent visits, none of the five successfully treated patients required continued antibiotic therapy or showed evidence of recurrent infection.
All eyes included in this study initially presented with corneal abscess associated with endophthalmitis. One patient had previously undergone keratoplasty and was included in the group with a successful outcome. Prior to the decision to implant the tube, all patients had received conventional endophthalmitis treatment, including intensive topical broad-spectrum antibacterial eye drops, oral antibiotic or antifungal therapy, anterior chamber lavage, conjunctival flap, vitrectomy, or keratoplasty.
Patient monitoring variables included best-corrected visual acuity (BCVA) before and after infection control. In most cases, preoperative BCVA was markedly reduced, ranging from light perception to 1/10. At 12 months postoperatively, BCVA remained unchanged in those with poor baseline vision. However, two patients achieved improved outcomes, with postoperative BCVA of 4/10 and 7/10, respectively.
Individual case descriptions
Individual case descriptions are provided below, while Table 1 presents a concise summary of the clinical characteristics, treatments, and outcomes of the series.
Case 1 (B.A.)
A 53-year-old woman, contact lens wearer, presented on April 15, 2021, with a one-week history of red eye and blurred vision. BCVA was 1/10 OD and 7/10 OS. Slit-lamp examination revealed a 5×5 mm corneal abscess in the right eye without hypopyon or Tyndall. The left eye was unremarkable. A corneal sample was taken for bacteriological, mycological, gram stain, culture, and antibiogram. Initial therapy included fortified vancomycin, ceftazidime, and amphotericin B. Despite keratectomy and conjunctival flap, the infection progressed with hypopyon and necrosis. On April 28, a silicone drainage tube was implanted from the anterior chamber to the suprascleral space, combined with intracameral antifungal lavage and conjunctivoplasty. Temporary improvement was followed by progressive keratoplasty necrosis and diffuse corneal opacification. Due to lack of resolution despite repeated lavages, evisceration was performed on May 26, 2021.
Case 2 (G.L.)
A patient with a history of keratoplasty in 2017 (OD) and 1999 (OS) for keratoconus underwent phacoemulsification with IOL implantation in the right eye on December 19, 2019. Immediate postoperative BCVA was light perception (LP). At 30 days, the right eye showed graft edema, Tyndall, inflammatory membrane, and a 3 mm hypopyon. Anterior chamber lavage with amphotericin, amikacin, and vancomycin was performed with culture sampling. Post-operative progress was satisfactory. On day 2 post-surgery, yag laser was performed in the RE with 16 shots of 1.3 mj breaking the pre-lenticular inflammatory membrane. Initial improvement was noted, though VA remained LP. Day 7 post-surgery, the IOP was 39 mmHg. On January 28, 2020, a silicone tube was implanted in the superonasal anterior chamber. The postoperative course was stable, without recurrent infection. At last follow-up, VA was LP, with diffuse graft opacity and tube in correct position.
Case 3 (C.A.)
On July 27, 2019, a patient presented with a corneal abscess in the left eye. VA was 20/200, not improving with correction. Slit-lamp examination showed a 5×5 mm central-inferior abscess with inflammatory edema and conjunctival injection. Fortified amphotericin B, amikacin, and vancomycin were initiated after cultures. With no improvement, a conjunctival flap was performed on August 22nd, 2019. Persistent infection led to penetrating keratoplasty with suprascleral tube placement on October 22nd, 2019. The immediate postoperative course was stable under antifungal and antibiotic therapy. During follow-up, infection resolved, although keratoplasty opacity developed, requiring retransplantation in January 2021. At last evaluation, VA in the left eye was counting fingers.
Case 4 (A.A.)
A patient presented on March 5th, 2012, after a foreign body injury to the right eye. Initial VA was 1/10 OD and 5/10 OS. Examination revealed keratitis with a corneal ulcer, without hypopyon. Cultures grew Staphylococcus epidermidis. Despite intensive fortified topical therapy and multiple anterior chamber lavages with broad-spectrum antibiotics and antifungals, the condition progressed to corneal melting. A conjunctival flap and subsequently a suprascleral silicone tube were placed on April 7th, 2012. Persistent abscess required penetrating keratoplasty, followed by repeat grafting due to failure. An amniotic membrane flap was performed to cover a non-healing ulcer. At final follow-up in December 2013, VA was doubtful LP with staphyloma and graft opacity, but without signs of active infection.
Case 5 (M.M.S.)
On April 6th, 2012, a patient was diagnosed with a 6 mm corneal abscess in the right eye. Initial therapy included topical gatifloxacin, followed by penetrating keratoplasty on June 6th, 2012. Postoperatively, VA improved to 6/10. In July, recurrence with Tyndall, fibrin, and hypopyon was observed despite fortified antibiotics and repeated anterior chamber lavages. On August 25th, 2012, a suprascleral silicone tube was placed in the conjunctival pocket, with the exit covered by a scleral patch. Additional antifungal therapy (itraconazole, amphotericin) was introduced. Later procedures included lens aspiration and synechiae lysis with vitrectomy. By late 2014, VA had stabilized at 20/25 with correction, with a clear graft and no evidence of infection, and the tube remained in good position.
Case 6 (H.C.)
A contact lens user was referred on March 15th, 2019, for a corneal abscess in the left eye. Initial VA was 4/10 OD and 7/10 OS with correction. Examination showed a 2×2 mm deep corneal abscess with stromal edema and AC cells. Cultures revealed unclassified fungi, and natamycin was started. Despite initial healing, recurrence occurred in April with a new abscess (5×5 mm) involving the visual axis. Multiple antifungal regimens and lavages were attempted. On June 3st, 2019, penetrating keratoplasty was performed, followed by repeated episodes of graft infection and inflammation. On July 16th, 2019, a suprascleral silicone tube was placed with conjunctival flap. Subsequent treatments included retransplantation and systemic antifungals. Over the following year, graft stability improved gradually. By 2021, the patient had a clear graft, pseudophakia after cataract surgery, and corrected VA of 4/10 OS, with no signs of infection.
Table 1. Summary of case series: refractory endophthalmitis treated with adjunctive suprascleral silicone tube implantation.
Case |
Patient |
Eye |
Clinical background |
Initial presentation |
Prior treatments |
Tube placement |
Outcome |
1 |
B.A. |
OD |
Contact lens user |
5×5 mm corneal abscess, ↓VA |
Fortified antibiotics, keratectomy, conjunctival flap |
04/28/21 |
Persistent infection → evisceration (26/05/21) |
2 |
G.L. |
OD |
Keratoplasty 2017 (OD), keratoplasty 1999 (OS), phaco+IOL 2019 |
Post-phaco endophthalmitis, VA LP, hypopyon |
AC lavage with antifungal + antibiotics, YAG laser, fortified drops |
01/28/20 |
Stable, VA LP, diffuse graft opacity, no infection |
3 |
C.A. |
OS |
Corneal abscess (2019) |
5×5 mm central-inferior abscess, VA 20/200 |
Fortified AB/antifungal, conjunctival flap, keratoplasty |
10/22/19 |
Infection resolved, retransplant 2021, VA CF |
4 |
A.A. |
OD |
Foreign body trauma (2012) |
Corneal ulcer, keratitis, later melting |
Fortified AB/antifungal, multiple AC lavages, conjunctival flap, keratoplasty ×2, amniotic flap |
04/07/12 |
Unsuccessful grafts, VA doubtful LP, no infection |
5 |
M.M.S. |
OD |
Corneal abscess (2012) |
6 mm corneal abscess, AC cells |
Fortified AB, keratoplasty, multiple AC lavages |
08/25/12 |
Good outcome, VA 20/25 with correction, clear graft |
6 |
H.C. |
OS |
Contact lens user (2019) |
Recurrent corneal abscess, VA 4/10 |
Fortified AB/antifungal, keratoplasty ×2, conjunctival flap, systemic antifungal |
07/16/19 |
Clear graft, pseudophakia, VA 4/10 (2021), no infection |
Discussion
Endophthalmitis remains one of the most challenging infectious conditions to manage in ophthalmology. Its complexity depends on multiple factors, including the clinical characteristics of the affected eye, patient adherence to treatment, and the virulence of the pathogen1-2. A severe complication is the potential spread of infection to the central nervous system, leading to meningoencephalitis through the anatomical continuity of the optic nerve with the intracranial cavity10. In certain cases, early evisceration may be required to prevent this life-threatening outcome11.
In general medicine, drainage is a well-established strategy for the management of abscesses, as it promotes resolution by removing infectious material that cannot be adequately penetrated by antimicrobial therapy3-7. Similarly, current treatments for corneal abscesses associated with endophthalmitis include intensive topical broad-spectrum antibiotics, systemic agents (though with limited intraocular penetration), anterior chamber lavage, vitrectomy, conjunctival flaps, and corneal transplantation12-15. However, these approaches may not always suffice in refractory cases.
The technique described in this study involves implanting a silicone tube to drain the anterior chamber, directing the aqueous humor into the suprascleral space. In this location, the fluid may come into contact with inflammatory cells from conjunctival vasculature, potentially enhancing immune clearance. To avoid conjunctival erosion from direct tube exposure, the device must be covered with a scleral or corneoscleral patch, subsequently overlaid by conjunctiva to ensure protection and functionality.
In our series, 5 of 6 patients achieved infection control using this procedure, suggesting that this approach can be performed safely as an adjunctive measure. Nonetheless, endophthalmitis can remain refractory due to multiple factors, and therefore complete resolution cannot be expected in all cases.
This study has several limitations. First, it was retrospective in design and included a small number of cases, which restricts the generalizability of the findings. Second, the heterogeneity of clinical presentations and prior treatments makes it difficult to establish standardized criteria for patient selection. However, this same diversity may also be considered a strength, as the procedure proved effective in most cases despite differences in etiology, previous interventions, and clinical course.
To our knowledge, there are no previous reports describing the use of a silicone tube device for anterior chamber drainage as an adjunctive treatment for refractory endophthalmitis. This makes the present series one of the first contributions in the field. Moreover, the detailed case-by-case analysis with systematic follow-up provides valuable insights into the potential utility and safety of this novel approach.
Conclusion
Anterior chamber drainage using a silicone tube device achieved infection control in 83% of cases in this series, demonstrating its potential as a safe and effective adjunctive strategy for the management of refractory endophthalmitis. This technique should not be seen as a replacement for established treatments, but rather as a complementary therapeutic tool within the current armamentarium against endophthalmitis. By offering an additional option in extreme scenarios, it may help preserve the globe and reduce the need for more radical interventions such as evisceration. Future prospective and multicenter studies with larger cohorts are warranted to validate these findings and to better define its role in clinical practice.
References ya lo vio gerard
1. Relhan N, Forster RK, Flynn HW Jr. Endophthalmitis: Then and Now. Am J Ophthalmol 2018; 187: xx-xxvii. doi: 10.1016/j.ajo.2017.11.021.
2. Wade CI, Whitescarver TD, Ashcroft CR, Hobbs SD, Purt B, Reddy AK, Colyer MH, Justin GA. Endophthalmitis: a bibliographic review. Int Ophthalmol 2021; 41(12): 4151-4161. doi: 10.1007/s10792-021-01967-y.
3. El Boghdady M, Ewalds-Kvist BM, Zhao S, Najdawi A, Laliotis A. Post-operative antibiotics for cutaneous abscess after incision and drainage: Variations in clinical practice. Access Microbiol 2022; 4(10): acmi000441. doi: 10.1099/acmi.0.000441.
4. Chin YK, Asokkumar R. Endoscopic ultrasound-guided drainage of difficult-to-access liver abscesses. SAGE Open Med 2020; 8: 2050312120921273. doi: 10.1177/2050312120921273.
5. Strong SM, Lazanakis SM, Ball E. Pelvic abscess - to drain or not to drain? Curr Opin Obstet Gynecol 2023; 35(5): 420-425. doi: 10.1097/GCO.0000000000000897.
6. Abulhasan YB, Al-Jehani H, Valiquette MA, McManus A, Dolan-Cake M, Ayoub O, Angle M, Teitelbaum J. Lumbar drainage for the treatment of severe bacterial meningitis. Neurocrit Care 2013; 19(2): 199-205. doi: 10.1007/s12028-013-9853-y.
7. Ren S, Luo Y, Shen X, Wu Q, Wu X, Ma C, Xiong Z, Gong R, Liu Z, Chen J, Wang W. Vacuum Sealing Drainage against Surgical Site Infection after Intracranial Neurosurgery. Surg Infect (Larchmt) 2024; 25(10): 728-736. doi: 10.1089/sur.2024.032.
8. Maldonado-Bas A, Maldonado-Junyent A, Maldonado-Junyent A. Microdevice for aqueous humor drainage Maldonado Bas -Pförtner. Arq Bras Oftalmol 2011; 74(3): 201-206. doi: 10.1590/s0004-27492011000300011.
9. Maldonado-Junyent A, Maldonado-Bas A, Gonzalez A, Pueyrredón F, Maldonado-Junyent M, Maldonado-Junyent A, Rodriguez D, Bulacio M. Oculo-peritoneal shunt: draining aqueous humor to the peritoneum. Arq Bras Oftalmol 2015; 78(2): 123-125. doi: 10.5935/0004-2749.20150032.
10. Rocha-de-Lossada C, Díaz Antonio T, Rachwani Anil R, Cuartero Jiménez E. Meningoencephalitis due to endogenous endophthalmitis by Klebsiella pneumoniae in a diabetic patient. Indian J Ophthalmol 2020; 68(7): 1456. doi: 10.4103/ijo.IJO_2016_19 (Errata publicada en: Indian J Ophthalmol 2020; 68(10): 2331. doi: 10.4103/0301-4738.295754).
11. Dave TV, Dave VP, Sharma S, Karolia R, Joseph J, Pathengay A, Pappuru RR, Das T. Infectious endophthalmitis leading to evisceration: spectrum of bacterial and fungal pathogens and antibacterial susceptibility profile. J Ophthalmic Inflamm Infect 2019; 9(1): 9. doi: 10.1186/s12348-019-0174-y.
12. Austin A, Lietman T, Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmology 2017; 124(11): 1678-1689. doi: 10.1016/j.ophtha.2017.05.012.
13. Viriya E, Mah F. Postrefractive infectious keratitis: prevention, diagnosis, management, and prognosis. Curr Opin Ophthalmol 2021; 32(4): 309-314. doi: 10.1097/ICU.0000000000000775.
14. Xie T, Jing M. Haemophilus influenzae-induced corneal ulcer and endophthalmitis triggered by hair dye. J Craniofac Surg 2022; 33(6): e545-e546. doi: 10.1097/SCS.0000000000008321.
15. Ali M, Dun C, Chen A, Saeed S, Prescott CR, Makary MA, Srikumaran D, Woreta FA. Early endophthalmitis rates and risk factors after corneal transplant surgeries in medicare beneficiaries from 2016 to 2019. Cornea 2024; 43(6): 676-684. doi: 10.1097/ICO.0000000000003403.