ORIGINAL ARTICLES
Visual acuity in high astigmatic eyes in Salta, Argentina
Rubén D. Danzaa, Abel Szepsb, Rafael Iribarrenc, Carla C. Lancad-e
a Departamento de Oftalmología, Hospital de Emergencias Carlos G. Durand, Buenos Aires, Argentina.
b Centro Oftalmológico Liniers, Buenos Aires, Argentina.
c Doctors Iribarren Eye Consultants, Buenos Aires, Argentina.
d Escola Superior de Tecnologia da Saúde de Lisboa (ESTeSL), Instituto Politécnico de Lisboa, Lisboa, Portugal.
e Comprehensive Health Research Center (CHRC), Escola Nacional de Saúde Pública, Universidade Nova de Lisboa, Lisboa, Portugal.
Oftalmol Clin Exp (ISSNe 1851-2658)
2024; 17(3): e385-e393.
DOI:10.70313/2718.7446.v17.n03.346
Abstract
Purpose: To identify factors that affect uncorrected visual acuity (UCVA) in highly astigmatic eyes.
Methods: This clinical study was performed at an Ophthalmology Clinic in the Northwest region of Argentina. The study population consisted of all consecutive patients, aged between 10 and 40 years, with bilateral astigmatism higher than 2 dioptres (D) in at least one eye. The protocol included the following tests: UCVA, pinhole VA, best corrected VA (BCVA), keratometry, scotopic pupil diameter measurement, subjective refraction, and objective refraction with cycloplegia.
Results: A total of 405 patients were included in the study. Their mean age was 22.04 ± 10.62 years and 52.3% were female (n=212). The mean SE was -1.53 ± 1.79 D and mean scotopic pupil was 5.4 ± 0.3 mm. In multiple linear logistic regression analysis, excluding amblyopes, worse pinhole VA (Odds ratio [OR]: 8.19, 95% confidence intervals [CI]: 4.76 to 14.07; p<0,001) and higher scotopic pupil size were significantly associated with worse UCVA. The presence of amblyopia was associated with higher pupillary diameter (OR: 4.37; 95% CI: 1.60 to 11.97; p=0.004).
Conclusions: Our study revealed that pupil size affects UCVA in astigmatic eyes of subjects living in a rural environment. Thus, further studies are necessary to understand if the correction of astigmatism should take in consideration pupil size, pinhole VA and UCVA.
Keywords: high astigmatism, refraction, pupil size, epidemiology.
Agudeza visual en ojos con alto astigmatismo en Salta, Argentina
Resumen
Objetivo: Identificar los factores que afectan a la agudeza visual no corregida (AVNC) en ojos con astigmatismo alto.
Método: Este estudio clínico se realizó en una clínica oftalmológica de la región noroeste de la Argentina. La población de estudio consistió en todos los pacientes consecutivos de entre 10 y 40 años de edad con astigmatismo bilateral superior a 2 dioptrías (D) en al menos un ojo. El protocolo incluyó pruebas de agudeza visual con y sin corrección y estenopeica, queratometría, diámetro pupilar escotópico, refracción subjetiva y refracción objetiva con cicloplejía.
Resultados: Un total de 405 pacientes fueron incluidos en el estudio. Su edad media era de 22,04 ± 10,62 años y el 52,3% eran mujeres (n=212). El equivalente esférico medio fue de -1,53 ± 1,79 D y la pupila escotópica media fue de 5,4 ± 0,3 mm. En el análisis de regresión logística lineal múltiple, excluyendo a los ambliopes, una peor AV estenopeica (Odds ratio[OR]: 8,19; intervalos de confianza del 95% [CI]: 4,76 a 14,07; p<0,001) y un mayor tamaño de la pupila escotópica se asociaron significativamente con una peor AVC. La presencia de ambliopía se asoció con un mayor diámetro pupilar (OR:4,37; IC 95%: 1,60 a 11,97; p=0,004).
Conclusiones: Este estudio reveló que el tamaño pupilar afecta a la AVNC en ojos astigmáticos de sujetos que viven en un entorno rural. Por lo tanto, son necesarios más estudios para comprender si la corrección del astigmatismo debe tener en cuenta el tamaño de la pupila, la AV estenopeica y la AVNC.
Palabras clave: astigmatismo alto, refracción, tamaño pupila, epidemiología.
Acuidade visual em olhos com alto astigmatismo em Salta, Argentina
Resumo
Objetivo: Identificar os fatores que afetam a acuidade visual não corrigida (AVNC) em olhos com alto astigmatismo.
Método: Este estudo clínico foi realizado em uma clínica oftalmológica da região noroeste da Argentina. A população do estudo consistiu em todos os pacientes consecutivos entre 10 e 40 anos de idade com astigmatismo bilateral maior que 2 dioptrias (D) em pelo menos um olho. O protocolo incluiu testes de acuidade visual com e sem correção e pinhole, ceratometria, diâmetro pupilar escotópico, refração subjetiva e refração objetiva com cicloplegia.
Resultados: Um total de 405 pacientes foram incluídos no estudo. A média de idade foi de 22,04 ± 10,62 anos e 52,3% eram mulheres (n=212). O equivalente esférico médio foi de -1,53±1,79 D e a pupila escotópica média foi de 5,4±0,3mm. Na análise de regressão logística linear múltipla, excluindo ambliopes, pior AV estenospeica (Odds ratio [OR]: 8,19; intervalo de confiança [IC] de 95%: 4,76 a 14,07; p<0,001) e maior tamanho da pupila escotópica foram significativamente associados a pior AVC. A presença de ambliopia foi associada ao maior diâmetro pupilar (OR: 4,37; IC 95%: 1,60 a 11,97; p=0,004).
Conclusões: Este estudo revelou que o tamanho da pupila afeta o AVNC em olhos astigmáticos de indivíduos que vivem em ambiente rural. Portanto, mais estudos são necessários para entender se a correção do astigmatismo deve levar em consideração o tamanho da pupila, a AV estenospeica e o AVNC.
Palavras-chave: astigmatismo elevado, refração, tamanho da pupila, epidemiologia.
Introduction
Astigmatism greater than 0.50 dioptres (D) accounts for approximately 13% of refractive errors in humans, and prevalence rates are as high as 30% or more, depending on region and ethnicity1-2. The presence of high degrees of astigmatism is associated with the development of amblyopia3-5. Additionally, an association between astigmatism and myopia has been observed6-7. In general, studies involving children of Asian and native American ancestry report a greater prevalence of high astigmatism compared to studies involving individuals of European, African and West Asian ancestry8-13. A study by Mohindra & Nagaraj compared the prevalence of high astigmatism of 3.00 D or more in primary school children consisting of native Canadians (northern Saskatchewan), Zuni and Navajo tribes, and European Canadians14. The results showed an unequal prevalence of astigmatism, with 27% in Zuni tribal members, 12.8% in Navajos, 7% in native Canadians (northern Saskatchewan) and 1.6% in European Canadians14.
The Northwest region of Argentina —where Salta is located— has been described as the region with the highest average proportion of native American ancestry in the country, ranging from 65% to 72%, comparable to other areas of South America, and similarly with high rates of astigmatism ranging from 15% to 30%, generally with-the-rule (WTR) astigmatism15-17. High astigmatism (≥ 2.00D) prevalence in this region was found to be 6%18. Astigmatism can lead to a decrease in uncorrected visual acuity (UCVA) and deterioration of quality of vision19. However, many subjects in the rural environment of Salta have high astigmatism and are not current users of refractive correction. The present study was developed to analyze the UCVA improvement of high astigmatic eyes by a pinhole effect. Additionally, the study analyzed the pinhole effect and pupil size in amblyopes.
Material and methods
The study population consisted of all consecutive patients, aged between 10 and 40 years, with bilateral astigmatism higher than 2 dioptres (D) in at least one eye, attending an ophthalmology consultation during a period of one year (between January 2021 and June 2022). The age range was chosen to include children and young adults that can answer accurately to subjective testing. Adults over 40 years of age were excluded to avoid the bias of hypermetropic refractive changes that occur at presbyopic years. Patients were excluded if they presented abnormalities of pupil or other pathologies, which could reduce visual acuity (VA), such as high myopia, keratoconus or other corneal degenerations, severe dry eye disease, cataract, glaucoma, vasculopathy, diabetic retinopathy or any other retinal pathology, as well as cognitive alterations or other central nervous system affections.
The study was conducted in accordance with the tenets of the Declaration of Helsinki and ethical approval was obtained from the Argentinean Council of Ophthalmology. Verbal consent was obtained from all subjects after the nature of the study was explained. Data were completely anonymized and in full compliance with data protection laws.
The protocol included the following tests: (1) monocular UCVA (Snellen optotype computer display at 3 meters distance; LG, Delphy, China), (2) monocular VA with pinhole (1.2 mm diameter), (3) monocular keratometry with autorefractometry (Huvitz, China), (4) subjective refraction and astigmatism was examined with Jackson cross cylinders, (5) monocular best corrected VA (BCVA). Additionally, refraction with cycloplegia was performed. Cycloplegia was performed with two drops of cyclopentolate 1% instilled in both eyes and the autorefractometry was performed 40 minutes later checking for non-responsive pupils. In cases where cycloplegic refraction results were different from subjective refraction the subject was scheduled for a new visit after one week (wash out period) to re-evaluate subjective refraction and BCVA. Spherical equivalent (SE) was calculated using the standard formula (SE = sphere +1/2*cylinder).
Outdoor and indoor Luxometry (UNI-T, UT383, Uni-Trend Tech, China) was performed on cloudy and clear days at the morning to find the regional illuminance during the study period.
Amblyopia was defined as a difference of one logMAR line between the best corrected vision of the best and worst eye. Anisometropia was defined by a difference of 1.00 D or more between the SE of both eyes and anisoastigmatism was defined by a difference of 1.00 D or more in the astigmatism of both eyes.
Statistical analysis
The results of numerical variables were expressed as means with their standard deviation or medians and interquartile range according to whether they had normal or skewed distribution. Discrete variables were expressed as absolute frequency and percentage. Descriptive statistics was performed for the main variables under study. Visual acuity was converted to LogMAR units for the analysis. For the comparison of numerical variables, the Student’s t-test or Mann Whitney U-test were used as appropriate. The right eye of each subject was chosen for analysis and comparison of UCVA ≤ 0.50 and > 0.50. Predictive factors were analyzed as continuous variables to verify the hypothesis that patients can have acceptable UCVA due to a pinhole effect of the pupil. We examined the association of predictive factors with UCVA using multiple logistic regression models, adjusting for confounders, such as age, sex, pinhole VA, BCVA, keratometry and SE with manual backward stepwise approaches. Additionally, we examined the association of the same predictive factors (excluding BCVA) with amblyopia. Any P ≤ 0.05 was considered statistically significant. All statistical analyses were carried out with SPSS (IBM, Armonk, NY, USA, version 27).
Results
This study involved 406 consecutive unselected patients with astigmatism equal or greater than 2.00 D in at least one eye, of whom one was excluded due to corneal scarring and keratoconus. A total of 405 patients were included in the study (Table 1). Their mean age was 22.04 ± 10.62 years and 212 were female (52.3%). The mean SE was -1.53 ± 1.79 D in the right eyes with no significant differences compared to the left eyes (p=0.58). The average refractive cylinder of the right eyes was -3.19 ± 1.39 D with a maximum of -7.00 D with no significant differences with the left eyes (p=0.13). The steepest and flattest keratometry of the right eyes were 41.47 ± 1.56 D (K1) and 44.52 ± 1.99 D (K2), with a difference of +3.05 ± 1.21 D (p<0.001). The difference between the subjective and keratometric astigmatism was small and not significant (+0.13 ± 1.43 D, p=0.13)) showing that there was virtually no internal astigmatism. The values for the left eye were similar (data not shown).
Sixty-nine per cent of the patients had myopic SE equal or less than -1.00 D and only 7.4% had hyperopic SE of +1.00 D or more. Only 14.3% had anisometropia greater than 1.00 D. In contrast, 17.5% had anisoastigmatism greater than 1.00 D. The UCVA in right eyes was 0.610.27 logMAR, rising to 0.38 ± 0.22 logMAR with the pinhole test. Mean BCVA was 0.03 ± 0.08 logMAR and it was similar for the right and left eyes.
Table 1. Patient demographics and characteristics including pupil size.
|
Mean ± SD |
Min |
Max |
Age (years) |
22.04 ± 10.62 |
10 |
40 |
Uncorrected visual acuity (LogMAR) |
0.61 ± 0.27 |
1.00 |
0.00 |
Pinhole visual acuity (LogMAR) |
0.38 ± 0.22 |
1.00 |
0.00 |
Best corrected visual acuity (LogMAR) |
0.03 ± 0.08 |
0.50 |
0.00 |
Scotopic pupil size (mm) |
5.40 ± 0.32 |
4.30 |
7.00 |
Keratometry (K1; D) |
41.47 ± 1.56 |
37.25 |
51.00 |
Keratometry (K2; D) |
44.52 ± 1.99 |
39.25 |
57.25 |
Spherical equivalent (D) |
-1.53 ± 1.79 |
-11.50 |
4.63 |
Values are means ± SD.
The mean scotopic pupil was 5.4 ± 0.3 mm. The luminance testing showed that outdoors in a cloudy day under the trees in the street there were 1730 lux, in a sunny day at the same location there were 3275 lux. Indoors in the examination room with lights on there were 244 lux, while near the place where autorefractometry and where scotopic pupil diameters were measured there were only 44 lux.
Subjects with UCVA > 0.50 LogMAR had significant worse pinhole VA by three lines. Additionally, there were significant differences in pupil size, keratometry (K2) and SE, although the differences were small (Table 2). In multiple linear logistic regression analysis, younger age and worse pinhole visual acuity were associated with worse UCVA (Table 3). When subjects with amblyopia were excluded from the analysis, age became non-significant (p=0.12). However, worse pinhole visual acuity remained significant (Odd Ratio [OR]: 8.19, 95% Confidence intervals [CI]: 4.76 to 14.07; p<0.001) and higher scotopic pupil size emerged as a significant variable associated with worse UCVA.
Table 2. Comparison of pupil size and other factors and mean uncorrected visual acuity.
|
UVA (LogMAR) |
P valuea |
|
≤ 0.50 (n=241) |
> 0.50 (n=163) |
|
|
Age (years) |
22.32 ± 10.82 |
21.53 ± 10.28 |
0.46 |
Pinhole visual acuity (LogMAR) |
0.25 ± 0.09 |
0.57 ± 0.21 |
<0.001 |
Best corrected visual acuity (LogMAR) |
0.01 ± 0.035 |
0.07 ± 0.11 |
<0.001 |
Scotopic pupil size (mm) |
5.35 ± 0.30 |
5.48 ± 0.34 |
<0.001 |
Keratometry (K1; D) |
41.52 ± 1.43 |
41.39 ± 1.74 |
0.43 |
Keratometry (K2; D) |
44.29 ± 1.75 |
44.87 ± 2.27 |
0.004 |
Spherical equivalent (D) |
-1.15 ± 0.99 |
-2.13 ± 2.42 |
<0.001 |
Values are means ± SD; aIndependent t-test.
Table 3. Association of key risk factors with uncorrected visual acuity (LogMAR).
Variables |
Unadjusted OR |
P value |
Multivariable OR |
P value |
Age (years), per 1 year ↑ |
0.99 (0.97-1.01) |
0.46 |
0.95 (0.91-1.00) |
0.046 |
Gender |
0.88 (0.59-1.31) |
0.53 |
0.78 (0.30-2.00) |
0.59 |
Pinhole visual acuity (LogMAR), per 0.05 ↑ |
6.44 (4.33-9.58) |
<0.001 |
7.84 (4.72-13.01) |
<0.001 |
Best corrected visual acuity (LogMAR), per 0.05 ↑ |
1.96 (1.57-2.45) |
<0.001 |
1.13 (0.78-1.64) |
0.51 |
Scotopic pupil size (mm), per 1 mm ↑ |
3.73 (1.91-7.28) |
<0.001 |
3.91 (0.97-15.81) |
0.056 |
Scotopic pupil size (mm), per 1 mm ↑* |
3.86 (1.85-8.05) |
<0.001 |
5.11 (1.15-22.73) |
0.032 |
Keratometry (K1; D), per 1 D ↑ |
0.95 (0.84-1.08) |
0.43 |
1.35 (0.78-2.34) |
0.28 |
Keratometry (K2; D), per 1 D ↑ |
1.16 (1.05-1.29) |
0.005 |
0.83 (0.54-1.29) |
0.41 |
Spherical equivalent (D), per 1 D ↑ |
0.71 (0.62-0.81) |
<0.001 |
0.84 (0.58-1.21) |
0.35 |
CI: Confidence intervals. OR: Odds ratio. ↑ Increase. *OR excluding subjects with amblyopia. Model included age, sex, pinhole visual acuity, best corrected visual acuity, scotopic pupil size, Keratometry 1, Keratometry 2 and spherical equivalent (R2=0.87).
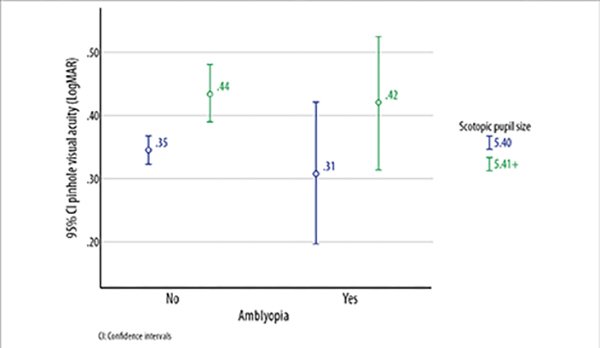
Amblyopia was present in 11.1% (n=45) of the subjects. Most subjects with amblyopia had anisometropia (n=20) or anisoastigmatism (n=12). Myopia was present in 22 of the 45 subjects with amblyopia. Subjects with amblyopia were significantly older, had worse BCVA and higher scotopic pupil size (Table 4). Figure 1 shows that subjects with higher scotopic pupil size had worse pinhole VA in both groups consisting of non-amblyopes and amblyopes.
Table 4. Comparison of pupil size and other factors and amblyopia.
|
Amblyopia |
P valuea |
|
|
No (n=360) |
Yes (n=45) |
|
Age (years) |
21.64 ± 10.63 |
25.20 ± 10.09 |
0.034 |
Uncorrected visual acuity (LogMAR) |
0.58 ± 0.26 |
0.62 ± 0.33 |
0.44 |
Pinhole visual acuity (LogMAR) |
0.38 ± 0.21 |
0.37 ± 0.25 |
0.83 |
Scotopic pupil size (mm) |
5.38 ± 0.32 |
5.54 ± 0.34 |
0.003 |
Keratometry (K1; D) |
41.43 ± 1.53 |
41.73 ± 1.80 |
0.30 |
Keratometry (K2; D) |
44.49 ± 1.91 |
44.75 ± 2.59 |
0.53 |
Spherical equivalent (D) |
-1.53 ± 1.72 |
-1.56 ± 2.22 |
0.93 |
Mean ± SD; aIndependent t-test.
In a binary logistic regression analysis the presence of amblyopia was associated with older age (OR:1.04; 95% CI: 1.01 to 1.08; p=0.013), worse UCVA (OR:1.17; 95% CI: 1.09 to 1.26; p<0.001) and higher scotopic pupillary diameter (OR:4.37; 95% CI: 1.60 to 11.97; p=0.004). On the other hand, better pinhole VA (OR: 0.87; 95% CI: 0.78 to 0.97; p=0.011) was a protective factor from amblyopia.

Figure 1. Pinhole visual acuity, scotopic pupil size and amblyopia.
CI: Confidence intervals
Discussion
The results of the present study showed that patients with astigmatism of -2.00D or more with smaller scotopic pupil sizes have better mean UCVA than patients with larger pupil size. Those findings are in accordance with previous studies showing that pupil size plays an important role in visual activities of daily life19-20. Additionally, mean VA with pinhole in patients from the present study improved more than two lines, from 0.61 ± 0.27 LogMAR to 0.38 ± 0.22 LogMAR. Previous studies have shown that pupil size affects visual acuity with defocus and astigmatism19, 21-23. Pinhole Visual Acuity was better in patients with LogMAR UCVA ≤ 0.50. We hypothesize that mean VA with pinhole may be similar to the mean UCVA these patients can achieve in an outdoor environment. Outdoor environments are characterized by high outdoor light illuminance levels. With higher amounts of light, the pupil size constricts to reduce of light scatter and retinal illuminance, improving visual acuity24. The discrimination of fine details improves with small pupil sizes by increasing the resolution of high spatial frequencies25. Typically reading tasks require a VA of 20/4026-27. In the present study, the mean unaided monocular VA was 20/80, raising to 20/50 with pinhole or possibly with high illumination in outdoor environment. Pupils adjust to provide optimal visual image quality28, and this may be the reason why many subjects reported not using spectacles for other purposes than reading or driving cars and attended the office appointment without wearing their spectacles. These results have important clinical implications, as further studies are necessary to understand if correction of pre-existing astigmatism in adults, to achieve better visual outcomes, in rural areas where people spend most of the day outdoors, is only necessary in eyes with larger pupils or UCVA > 0.50. It is also important to understand if pupils natural adjust to their astigmatism. Further studies should further explore natural adaptation to defocus as suggested by Campbell et al and others28-29.
In children, pupil size may also be involved in the emmetropization process due to its contribution to the angular spread across the retinal thickness for highest visual acuity both for foveal and parafoveal vision30. Thus, further longitudinal studies are necessary to confirm the role of pupil size in refractive error development as we found that amblyopes have higher scotopic pupil size compared with non-amblyopes. Research on pupil size differences in amblyopia is sparse31-32. One previous study reported that higher hyperopic eyes with amblyopia and fellow eyes had statistically significantly lower scotopic pupil diameter values compared with healthy eyes33. Additionally, previous research on refractive errors and pupil size found that myopes have higher pupil size24. However, there are contradictory reports34. Inner retinal photoreceptors control pupil response, which may explain the process of amblyopia development. The Inner retinal photoreceptors and the melanopsin activity may be involved in a combined mechanism that reacts to light and adjust to pathological changes. Further longitudinal studies may shed light on the relationship between amblyopia and pupil size.
There are several limitations to this study. We only measured high-contrast monocular VA in indoor illumination conditions, and the measurements in this study may not reflect natural binocular viewing conditions outdoors. Pupil size was not measured in the same environment used for visual acuity measures, which can influence the pupil size as light levels may change and vary between observers. Additionally, in further studies a dark adaptation period to remove the lingering impact of cone activity on pupil is necessary. The cross-sectional design precludes an evaluation of whether pupil size responses changed due to UCVA.
Conclusion
Our study revealed that pupil size can affect VA, especially in astigmatic eyes with UCVA ≤ 0.50 in patients living in a rural environment with high light intensity outdoors. Thus, further studies are important to shed light on whether the correction of astigmatism in adults should take in consideration the amount of astigmatism, pupil size and UCVA. In our study, eyes with larger pupils or UCVA > 0.50, needed correction to achieve better visual outcomes. These findings may be clinically relevant as most surgeons only consider the amount and axis of astigmatism for the surgical correction in clinical settings.
References
1. Saw SM, Goh PP, Cheng A et al. Ethnicity-specific prevalences of refractive errors vary in Asian children in neighbouring Malaysia and Singapore. Br J Ophthalmol 2006; 90: 1230-1235.
2. Kleinstein RN, Jones LA, Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error Study Group et al. Refractive error and ethnicity in children. Arch Ophthalmol 2003; 121: 1141-1147.
3. Brown SA, Weih LM, Fu CL et al. Prevalence of amblyopia and associated refractive errors in an adult population in Victoria, Australia. Ophthalmic Epidemiol 2000; 7: 249-258.
4. Abrahamsson M, Sjöstrand J. Astigmatic axis and amblyopia in childhood. Acta Ophthalmol Scand 2003; 81: 33-37.
5. Dobson V, Miller JM, Harvey EM, Mohan KM. Amblyopia in astigmatic preschool children. Vision Res 2003; 43: 1081-1090.
6. Fulton AB, Hansen RM, Petersen RA. The relation of myopia and astigmatism in developing eyes. Ophthalmology 1982; 89: 298-302.
7. Farbrother JE, Welsby JW, Guggenheim JA. Astigmatic axis is related to the level of spherical ametropia. Optom Vis Sci 2004; 81: 18-26.
8. Harvey EM, Dobson V, Miller JM et al. Prevalence of corneal astigmatism in Tohono O'odham Native American children 6 months to 8 years of age. Invest Ophthalmol Vis Sci 2011; 52: 4350-4355.
9. Emerole CG NR, Osim EE. Astigmatism: prevalence, distribution and determinants in Owerri, Nigeria. J Exp Clin Anat 2013; 12: 87-91.
10. Wajuihian SO. Characteristics of astigmatism in black South African high school children. Afr Health Sci 2017; 17: 1160-1171.
11. Wang J, Cheng QE, Fu X et al. Astigmatism in school students of eastern China: prevalence, type, severity and associated risk factors. BMC Ophthalmol 2020; 20: 155.
12. Chebil A, Jedidi L, Chaker N et al. Characteristics of astigmatism in a population of Tunisian school-children. Middle East Afr J Ophthalmol 2015; 22: 331-334.
13. Hashemi HAS, Yekta AA, Ostadimoghaddam H et al. Prevalence of Astigmatism in 4 to 6 Year-Old Population of Mashhad, Iran. J Compr Ped 2015; 6: 1-5.
14. Mohindra I, Nagaraj S. Astigmatism in Zuni and Navajo indians. Am J Optom Physiol Opt 1977; 54: 121-124.
15. Hashemi H, Fotouhi A, Yekta A et al. Global and regional estimates of prevalence of refractive errors: Systematic review and meta-analysis. J Curr Ophthalmol 2018; 30: 3-22.
16. Lira RP, Santo IF, Astur GL et al. Refractive error in school children in Campinas, Brazil. Arq Bras Oftalmol 2014; 77: 203-204.
17. Thorn F, Cruz AA, Machado AJ, Carvalho RA. Refractive status of indigenous people in the northwestern Amazon region of Brazil. Optom Vis Sci 2005; 82: 267-272.
18. Zeman L, Danza RD, Fejerman L, Iribarren R. Prevalence of high astigmatism in Salta province, Argentina. Oftalmol Clin Exp 2021; 14: 162-170.
19. Kamiya K, Kobashi H, Shimizu K et al. Effect of pupil size on uncorrected visual acuity in astigmatic eyes. Br J Ophthalmol 2012; 96: 267-270.
20. Atchison DA, Smith G, Efron N. The effect of pupil size on visual acuity in uncorrected and corrected myopia. Am J Optom Physiol Opt 1979; 56: 315-323.
21. Watanabe K, Negishi K, Dogru M et al. Effect of pupil size on uncorrected visual acuity in pseudophakic eyes with astigmatism. J Refract Surg 2013; 29: 25-29.
22. Singh A, Pesala V, Garg P, Bharadwaj SR. Relation between uncorrected astigmatism and visual acuity in pseudophakia. Optom Vis Sci 2013; 90: 378-384.
23. Knorz MC, Koch DD, Martinez-Franco C, Lorger CV. Effect of pupil size and astigmatism on contrast acuity with monofocal and bifocal intraocular lenses. J Cataract Refract Surg 1994; 20: 26-33.
24. Maqsood F. Effects of varying light conditions and refractive error on pupil size. Cogent Medicine 2017; 4: 1-7.
25. Eberhardt LV, Strauch C, Hartmann TS, Huckauf A. Increasing pupil size is associated with improved detection performance in the periphery. Atten Percept Psychophys 2022; 84: 138-149.
26. Vejarano F, Alió J, Iribarren R, Lança C. Non-miotic improvement in binocular near vision with a topical compound formula for presbyopia correction. Ophthalmol Ther 2023; 12: 1013-1024.
27. Sanders DR, Sanders ML. Near visual acuity for everyday activities with accommodative and monofocal intraocular lenses. J Refract Surg 2007; 23: 747-751.
28. Campbell FW, Gregory AH. Effect of size of pupil on visual acuity. Nature 1960; 187: 1121-1123.
29. Cakmak HB, Cagil N, Simavli H et al. Refractive error may influence mesopic pupil size. Curr Eye Res 2010; 35: 130-136.
30. Vohnsen B, Sharmin N, Qaysi S, Rodriguez Rodriguez MI. The optical role of pupil size in eye growth and emmetropia Invest Ophthalmol Vis Sci 2019; ARVO Annual Meeting Abstract, July 2019.
31. González de Aledo Linos A. Anisocoria: un signo de sospecha de anisometropía y/o ambliopía. An Esp Pediatr 1991; 34: 9-14.
32. Kocamış SL, Çağil N. Investigation of the pupil diameter differences in anisometropic amblyopia. Turk J Ophthalmol 2013; 43: 45-50.
33. Yetkin E, Tekin K, Kiziltoprak H et al. Evaluation of static and dynamic pupil characteristics in hyperopic anisometropic amblyopia. Eur J Ophthalmol 2019; 29: 486-493.
34. Kiziltoprak H, Tekin K, Yetkin E, Sekeroglu MA. Static and dynamic pupil characteristics in myopic anisometropic amblyopia. Beyoglu Eye J 2020; 5: 86-92.