SCIENTIFIC OPINIONS
Evidence-based ophthalmology: the new “6S” evidence pyramid
Joaquín Fernández
QVision, Hospital Vithas Almería, Almería, España.
Oftalmol Clin Exp (ISSNe 1851-2658)
2024; 17(2): e166-e173.
Part of this material has been previously published in QVision’s blog: https://www.qvision.es/blogs/joaquin-fernandez/2017/07/30/6-12-oftalmologia-basada-evidencia-la-nueva-piramide-de-la-evidencia-6s/
Abstract
Scientific knowledge evolves rapidly, demanding that physicians stay updated on new diagnostic and therapeutic methods, considering factors like cost and accessibility to improve medical services and patient care quality. Choosing reliable information efficiently is a major challenge for doctors. Understanding research study designs is crucial, with the 6S pyramid simplifying this process by aiding in selecting the most informative material quickly. However, knowledge generation at higher levels of the pyramid relies on prior construction at lower levels. Some topics may only have evidence available at lower levels due to their novelty, necessitating risk management in decision-making. Artificial intelligence holds promise for aiding medical decision-making, potentially enabling quicker and more accurate decisions regardless of physicians’ experience. Overall, mastering evidence-based medicine tools is essential for informed decision-making and effective medical practice.
Keywords: evidence-based medicine, evidence hierarchy, evidence pyramid, medical risk management, artificial intelligence.
Introduction
Scientific knowledge is dynamic and fast-paced. Medicine requires physicians to stay updated on new diagnostic and therapeutic methods, while also considering aspects related to healthcare costs and accessibility, all of which impact the level of medical service and the quality of care patients receive. To choose which technology to acquire or which treatment to recommend, a major challenge for physicians is developing the ability to select information appropriately to make the right decisions. Selecting information sources that are time-efficient, accurate, reliable, and unbiased (or with explicitly declared conflicts of interest) is crucial.
This article will review some basic concepts of a visual tool we use in evidence-based medicine practice to prioritize information, mainly concerning study design. This allows a physician to choose one article over another as a source of information, saving time and obtaining the information required to make the best decision for each patient. However, this means that the physician must understand methodological aspects that define and differentiate, for example, a case report from a systematic review. We will also see how the new 6S pyramid model simplifies this process.
Evidence and hierarchies
The first and oldest principle of evidence-based medicine indicates a hierarchy of evidence that reflects the relative authority of different types of studies, which creates different levels of evidence. Given that evidence is described as a hierarchy, a compelling rationale was made for an evidence pyramid. Although there is broad agreement on the relative strength of the main types of epidemiological studies, there is no single, universally accepted hierarchy.

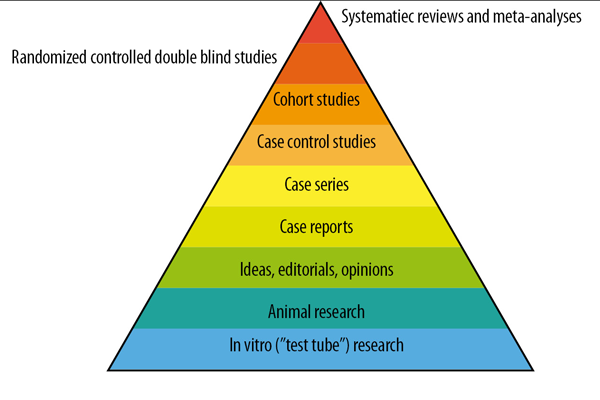
Figure 1. Traditional evidence pyramid, to establish levels of hierarchy of scientific information.
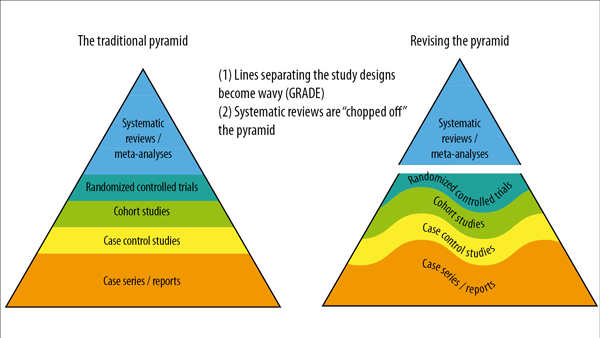
Figure 2. Visual modifications of the traditional pyramid where the straight lines separating the study designs become wavy lines, and systematic reviews are “cut” from the pyramid and used as a lens through which evidence is analyzed.

Figure 3. New evidence pyramid of the six “S” letters (6S pyramid
Traditional evidence pyramid
In 1995, Guyatt and Sackett published the first hierarchy of this kind1, and in 1997, Greenhalgh hierarchized different types of studies, showing weaker study designs at the base of the pyramid2: basic science, expert opinions, and case series, followed by case-control studies, cohort studies, randomized controlled trials (RCTs), and at the top, systematic reviews and meta-analyses. This description is intuitive and likely correct in many cases. Most versions of the pyramid clearly represented a hierarchy of internal validity (risk of bias), and some versions incorporated external validity (applicability) into the pyramid.
The traditional evidence pyramid was sometimes considered too simplistic, as it left no room to argue and counter-argue the methodological merit of different study designs3. Other potential weaknesses challenged the placement of systematic reviews and meta-analyses at the top of the pyramid. For example, the heterogeneity (clinical, methodological, or statistical) inherent in meta-analyses can be minimized or explained but never eliminated4. The methodological complexities of systematic reviews could generate uncertainty and bias5. An evaluation of 163 meta-analyses demonstrated that the estimation of treatment outcomes varied substantially depending on the analytical strategy used5.
Therefore, from this perspective, two visual modifications of the pyramid are suggested to illustrate two contemporary methodological principles6:
1. The straight lines separating the study designs become wavy lines
In the early 2000s, the “Grading of Recommendations Assessment, Development and Evaluation (GRADE)” developed a framework in which the certainty of evidence was based on numerous factors, not just the study design, challenging the pyramid concept. Certain methodological limitations of a study, imprecision, and inconsistency are factors independent of the study design that can affect the quality of the derived evidence. Therefore, the first modification of the pyramid was to change the straight lines separating the study designs into wavy lines (moving up and down to reflect the GRADE approach of rating up and down based on various levels of evidence quality).
2. Systematic reviews are “cut” from the pyramid and used as a lens through which evidence is analyzed
Another challenge to the notion of placing systematic reviews at the top of the evidence pyramid relates to the article presented in the Journal of the American Medical Association user’s guide on systematic reviews and meta-analyses. The guide presents a two-step approach in which the credibility of the systematic review process is first evaluated (comprehensive literature search, rigorous study selection process, etc.). If the systematic review is deemed sufficiently credible, then a second step is undertaken to assess the certainty of the evidence based on the GRADE approach7. However, a well-conducted meta-analysis of RCTs with a low risk of bias cannot be equated with a meta-analysis of observational studies with a higher risk of bias. Thus, the second modification to the pyramid was to remove systematic reviews (the study selection process) and meta-analyses (the statistical analysis of these) from the top of the pyramid and use them as a lens through which primary studies should be evaluated.
New evidence pyramid: the “6S” pyramid
For a time, applying the highest quality evidence to clinical decision-making involved searching the literature and using critical appraisal skills to distinguish lower-quality clinical studies from higher-quality ones. However, in the last decade, many practical resources have been created to facilitate easy access to high-quality research. We call these pre-appraised resources because they have undergone a filtering process to include only the highest quality studies, and they are regularly updated to ensure that the evidence accessed through these resources is as current as possible.
To facilitate the use of these pre-appraised resources, Haynes proposed a “4S” model8, which he later refined into a “5S” model9 and subsequently into a “6S” model10. The fundamental purpose of this hierarchy is to emphasize that the evidence sources at the base of the “6S” pyramid are less preferred in clinical practice because they require more expertise and time to identify, appraise, and apply.
From the base to the apex, they are individual studies, synopsis of individual studies, syntheses, synopsis of syntheses, summaries, and systems. We will rely on Haynes’ original article to explain the new “6S” evidence pyramid and another by Windish to see its practical application10-11.
Next, we will present the main concepts of each of the six levels of evidence.
First S: Individual studies
Clinical question: I want to find the latest treatment for my patient’s disease and I want to use an evidence-based approach (ask, acquire, appraise, and apply). Where should I look for this information?
Answer: In this case, it would be best to search the primary literature for individual articles.
Examples of resources: PubMed.
Strengths: The most up-to-date individual studies are available both online and in print. Many of these resources provide alerts when new articles are published on a topic of interest.
Weaknesses: When using individual studies, it is necessary to understand how to use search engines to find the study of interest and then interpret and apply the individual study on your own without expert opinión? This can be tedious and time-consuming.
Second S: Synopsis of individual study
Concept: A synopsis of a single study provides a brief but often sufficiently detailed summary of a high-quality study that can inform clinical practice of its applicability.
Clinical question: I want to find the latest treatment for my patient’s disease but: I don't have time to read the study thoroughly, and/or I don’t feel that I have the expertise to interpret the information in the article. Where should I look for this information?
Answer: Using resources that summarize individual studies would be the best place to look. Some of these resources include literature evaluation and application.
Examples of resources: Medicine Evidence-Based, Year Book of Ophthalmology.
Strengths: The advantages of a synopsis of a single study over the Individual study are threefold: the assurance that the study is of sufficiently high quality and clinical relevance to merit abstraction, the brevity of the summary, and the added value of commentary.
Weaknesses: Not all studies have a synopsis, so the list of studies with a summary is not extensive.
Third S: Synthesis
Concept: A synthesis or systematic review is a comprehensive summary of all research evidence related to a focused clinical question. It is a multi-step process in which the question is formulated, relevant studies are identified and assessed for study quality, data are extracted and quantitatively (in the form of meta-analysis) or non-quantitatively synthesized, and conclusions are drawn. The aim of synthesis is not to establish a recommendation but to provide an analysis of the current knowledge.
Clinical question: I know there is a lot of literature on my clinical question, but is there a resource I can use that integrates all this information and provides recommendations given the data?
Answer: systematic reviews with or without meta-analysis are ideal for these types of questions.
Examples of resources: PubMed, Cochrane Library, DynaMed, Database of Abstracts of Reviews of Effects (DARE), Campbell Library, Agency for Healthcare Research and Quality (AHRQ).
Strengths of systematic review:
Weaknesses of systematic review:
Fourth S: Synopsis of synthesis
Concept: Since many busy clinicians do not have time to review detailed systematic reviews, a synopsis summarizing the results of a high-quality systematic review can often provide enough information to support clinical action.
Clinical question: I know there is a lot of literature on my question, but I don’t want to read an entire systematic review to determine the evidence. Where should I look for this information?
Answer: Use resources that provide a synopsis of synthesis.
Examples of resources: ACP Journal Wise, Evidence-Based Medicine, DARE, DynaMed, Journal Watch, AHRQ; Bandolier.
Strengths: The advantages of finding a relevant synopsis of synthesis from a systematic review are twofold: First, the synopsis of synthesis provides a summary of the corresponding systematic review, and second, it is often accompanied by commentary addressing the methodological quality of the synthesis and the clinical applicability of its conclusions.
Weaknesses: A limitation is that it takes time to prepare a Systematic Review after the publication of the original studies, and a Synopsis further extends this time.
Fifth S: Summaries
Concept: These include clinical guidelines or textbook summaries that integrate evidence-based information about specific clinical problems and provide periodic updates. Current evidence-based clinical practice guidelines (CPGs), which are “systematically developed statements to assist practitioner and patient decisions about appropriate health care for specific clinical circumstances”11-12, are also examples of evidence at the “summary” level. A CPG should be based on comprehensive literature searches and evaluations (ideally current systematic reviews, if available), and each recommendation should be accompanied by its level of evidence. Healthcare professionals should consider acting only on those recommendations based on high-quality evidence.
Clinical question: I just want to apply evidence to my patients when the issue arises. Where can I find clinical outcomes?
Answer: Look for summaries that provide evidence-based decision clinical practice guidelines.
Examples of resources: National Guidelines Clearinghouse, Clinical Evidence, ACP’s Physicians’ Information and Education Resource (PIER), UpToDate, AHRQ, Skinsight, GIDEON, Preferred Practice Pattern® Guidelines of American Academy of Ophthalmology.
Strengths: These summaries often provide levels of evidence to help determine the strength of the evidence for a particular therapy, screening, etc.
Weaknesses: Summaries must be updated frequently as evidence changes. Depending on the organization setting the guidelines, different clinical practice guidelines may exist. Therefore, it may be challenging to decide on best practices based on different guidelines.
Sixth S: Systems
Concept: An evidence-based clinical information system integrates and concisely summarizes all relevant and important research evidence on a clinical problem, updates as new research evidence becomes available, and automatically links (via an electronic medical record) a patient’s specific circumstances to relevant information8. In these computerized decision support systems (CDSS) detailed individual patient data are inputted into a computer program and combined with software or algorithms in a computerized knowledge base, resulting in the generation of specific assessments or recommendations for clinicians. For example, CDSSs exist for managing oral anticoagulation in primary care led by nurses in the UK13 and for increasing influenza vaccination in hospitalized patients14.
Clinical question: How can I follow guidelines for each of my patients?
Answer: Electronic health records that have computerized decision support systems (CDSS) would be a way to keep each patient’s care updated and evidence-based in real-time.
Examples of resources: These systems are evolving and currently not widespread. The most common example is observed in the electronic medical record of the US Department of Veterans Affairs.
Strengths: A system would include a personalized health plan for each patient based on their individual characteristics and health status.
Weaknesses: The system itself would need to be regularly updated as evidence-based medical decisions change. One potential drawback is that a system may not be able to consider patient preferences in decision-making.
For readers with limited time, using search engines to help find the best available evidence can be useful. Sites like Google have a custom search link that can be utilized. Google also has Google Scholar which can assist with medical literature searches. Databases that can be searched include the TRIPDATABASE, Evidence-Based Medicine Reviews, the Centre for Reviews and Dissemination, and SUMSearch 2. Unfortunately, some of these search engines and reviewed resources require a paid subscription.
Currently, we should use the best available evidence at the most specific level when searching medical literature. Developing proficiency with one or two resources at each step of the evidence pyramid can only help improve our efficiency and effectiveness during our clinical investigations.
Final concepts
Understanding evidence-based medicine tools is indispensable for practicing in today’s world and making appropriate decisions, independently. For this, it is necessary to understand at least the basic aspects of different research study designs. However, the system that hierarchizes levels of evidence in the 6S pyramid simplifies the process for the general physician to choose material that provides the most information in the shortest reading time, with the highest level of hierarchy, especially among the top three levels (S4 to S6). However, for knowledge generation at the upper levels of the S6 pyramid, prior knowledge construction is required at the lower levels (S1 to S3). Therefore, there may be topics that, due to their novelty, only have information available at S1 or S2 levels. The physician must manage the risks of making decisions when evidence levels are low. Finally, while artificial intelligence promises to be of great utility for our daily practice, its application at level S1 may have a significant impact on medical decision-making, which can be made in increasingly less time, more accurately, and also independently of the physician’s years of experience (as we will all be assisted uniformly by these computer systems).
References
1. Guyatt GH, Sackett DL, Sinclair JC et al. Users’ guides to the medical literature IX: a method for grading health care recommendations. Evidence-Based Medicine Working Group. JAMA 1995; 274: 1800-1804.
2. Greenhalgh T. How to read a paper: getting your bearings (deciding what the paper is about). BMJ 1997; 315: 243-246.
3. Vandenbroucke JP. Observational research and evidence-based medicine: what should we teach young physicians? J Clin Epidemiol 1998; 51: 467-472.
4. Berlin JA, Golub RM. Meta-analysis as evidence: building a better pyramid. JAMA 2014; 312: 603-605.
5. Dechartres A, Altman DG, Trinquart L et al. Association between analytic strategy and estimates of treatment outcomes in meta-analyses. JAMA 2014; 312: 623-630.
6. Murad MH, Asi N, Alsawas M, Alahdab F. New evidence pyramid. Evid Based Med 2016; 21: 125-127.
7. Murad MH, Montori VM, Ioannidis JP et al. How to read a systematic review and meta-analysis and apply the results to patient care: users’ guides to the medical literature. JAMA 2014; 312: 171-179.
8. Haynes, RB. Of studies, summaries, synopses, and systems: the “4S” evolution of services for finding current best evidence. Evid Based Ment Health 2001; 4: 37-39.
9. Haynes RB. Of studies, syntheses, synopses, summaries, and systems: the “5S” evolution of information services for evidence-based health care decisions. ACP J Club 2006; 145: A8.
10. DiCenso A, Bayley L, Haynes RB. ACP Journal Club. Editorial: Accessing preappraised evidence: fine-tuning the 5S model into a 6S model. Ann Intern Med 2009; 151: JC3-2, JC3-3.
11. Windish D. Searching for the right evidence: how to answer your clinical questions using the 6S hierarchy. Evid Based Med 2013; 18: 93-97.
12. Field MJ, Lohr KN, eds. Clinical practice guidelines: directions for a new program. Washington, DC: National Academy Press, 1990.
13. Fitzmaurice DA, Hobbs FD, Murray ET et al. Oral anticoagulation management in primary care with the use of computerized decision support and near-patient testing: a randomized, controlled trial. Arch Intern Med 2000; 160: 2343-2348.
14. Gerard MN, Trick WE, Das K et al. Use of clinical decision support to increase influenza vaccination: multi-year evolution of the system. J Am Med Inform Assoc 2008; 15: 776-779.