
CLINICAL CASES
Complication of device insertion of dexamethasone intravitreal implant: applicator OzurdexTM malfunction
Ekhiñe Unzalu Lerma, Eduardo Pérez-Salvador García
Department of Ophthalmology, Burgos University Hospital, Burgos, España.
Received: October 28th, 2023.
Approved: November 12nd, 2023.
Corresponsal author
Dra. Ekhiñe Unzalu Lerma
Hospital Universitario de Burgos
Avenida Islas Baleares, 3
09006 Burgos
España
+34 669 90 23 13
ekiunza@gmail.com
Oftalmol Clin Exp (ISSNe 1851-2658)
2023; 16(4): e428-e433.
Acknowledgments
Authors thank Luis Pérez-Salvador for the graphical images of this article.
Disclosures financial support
No financial support was received for this submission.
Conflict of interest
None of the authors has conflict of interest with this submission.
Abstract
Purpose: To report a non-pharmacological complication occurred with the applicator of the dexamethasone intravitreal implant.
Case report: Report a case of a 76-year-old pseudophakic women treated with dexamethasone intravitreal implant (Ozurdex™, Abbvie) because of cystoid macular edema in the left eye. During the procedure a malfunction related to the mandrel during the injection procedure occurred. The mandrel of the device came out of the pen, becoming embedded in the sclera. As soon as the bar was visualized in the sclera, it was removed with the help of Adson forceps. No complications occurred in the retina.
Conclusion: The mandrel of the device could be more damaging than the micronized dexamethasone rod and can be propelled out with sufficient force and speed if it impacts on the contralateral retina. This can occur —as it did in this case— because the mandrel is attached by a plastic portion of only 0.1 mm to the rest of the piece.
Key words: Ozurdex™, intravitreal dexamethason, cystoid macular edema, retina, complications.
Complicación con la inserción del dispositivo de implante intravítreo de dexametasona: mal funcionamiento del aplicador Ozurdex®
Resumen
Objetivo: Informar sobre un caso de una complicación no farmacológica relacionada con implante intravítreo de dexametasona.
Caso clínico: Se trata de una mujer pseudofáquica de 76 años con edema macular cistoide en el ojo izquierdo. Durante el procedimiento de inyección se produjo un mal funcionamiento del aplicador del liberador de dexametasona (Ozurdex®, Abbvie). El mandril del dispositivo se salió de la pluma y quedó incrustado en la esclerótica. Tan pronto como se observó la barra en la esclerótica, se retiró con ayuda de unas pinzas de Adson. No se produjeron complicaciones en la retina.
Conclusión: El mandril del dispositivo podría ser más dañino que la varilla de dexametasona micronizada y puede ser propulsado con suficiente fuerza y velocidad si impacta en la retina contralateral. Esto puede ocurrir —como sucedió en este caso— debido a que el mandril está unido por una porción de plástico de solo 0,1 mm al resto de la pieza.
Palabras clave: Ozurdex®, dexametasona intravítrea, edema macular cistoide, retina, complicaciones.
Complicação com a inserção do dispositivo de implante intravítreo de dexametasona: mau funcionamento do aplicador Ozurdex®
Resumo
Objetivo: Relatar um caso de complicação não farmacológica relacionada ao implante intravítreo de dexametasona.
Caso clínico: Trata-se de uma mulher pseudofácica de 76 anos com edema macular cistóide em olho esquerdo. Durante o procedimento de injeção, ocorreu mau funcionamento do aplicador liberador de dexametasona (Ozurdex®, Abbvie). O mandril do dispositivo saiu da caneta e ficou incrustado na esclera. Assim que a barra foi observada na esclera, ela foi removida com auxílio de pinça Adson. Não ocorreram complicações na retina.
Conclusão: O mandril do dispositivo pode ser mais prejudicial que a haste micronizada de dexametasona e pode ser impulsionado com força e velocidade suficientes se impactar a retina contralateral. Isso pode acontecer —como aconteceu neste caso— porque o mandril está preso por uma porção de plástico de apenas 0,1 mm ao restante da peça.
Palavras-chave: Ozurdex®, dexametasona intravítrea, edema macular cistóide, retina, complicações.
Introduction
Dexamethasone (DEX) intravitreal implant 0.7 mg (Ozurdex™, AbbVie) is a sustained-release, biodegradable, DEX containing implant approved by the Food and Drug Administration for the treatment of macular edema following branch retinal vein occlusion (BRVO) or central retinal vein occlusion, noninfectious posterior uveitis, and diabetic macular edema from diabetic retinopathy1-2. Although anti-vascular endothelial growth factor agents (bevacizumab, ranibizumab, aflibercept, faricimab) have revolutionized the treatment of these diseases, intravitreal steroid injections, including the DEX implant, have proven to be an effective treatment in refractive cases1-3.
Intravitreal drug injection implants are generally safe but do carry some risk, from both the procedure itself and adverse effects of the medications. Injection-related non-pharmacological complications included: retinal tear/detachment, vitreous loss, retinal and vitreous haemorrhage, endophthalmitis, implant segmentation, cataract secondary to lens injury, implant injection into the lens body, macular hole, subretinal migration and migration of the implant into the anterior chamber4-6.
Our purpose is to report a case of an non-pharmacological complication occurred with the applicator of the Ozurdex implanting device. To our knowledge, this is the fist study reporting this kind of intrasurgical incident.
Case report
A 76-year-old pseudophakic women presented cystoid macular edema (CEM) on the left eye. Pars plana vitrectomy (PPV) with epirretinal membrane peel was successfully performed in August 2018 because of an epiretinal membrane. Over the next years, the patient received three dexamethasone implants to control the CEM, but in the last one, during the process of intravitreal implant, a complication has occurred.
After opening the package of the Ozurdex™, the expiration date was checked (December 2025). The sealed foil pouch was removed from the carton and no damage was noticed. The nurse opened the foil pouch over a sterile field and gently dropped the applicator on a sterile surgical tray.
The procedure of the Ozurdex™ injection was conducted performing all the recommended steps to ensure surgical safety. After cutaneous povidone at 10%, eye sterile drapping, using an sterile eyelid speculum to facilite the maneuvers of implantation, administered topical anesthesic and povidone drops at 5% and subconjunctival anesthesia at site of injection at 3.5 mm from limbus to lower temporal, the cap was carefully removed from the applicator, then the safety tab was pulled straight off the applicator, and the tip of the needle entered bevel facing up obliquely at first advancing approximately 1 mm within the sclera and then directed toward the center of the eye, once all the needle was in vitreous cavity and the sleeve touched the conjunctiva, the actuator button was depressed slowly in order to release the implant and a audible click sound was heard as usual.
The mandrel of the dexamethasone-releasing device came out of the pen, becoming embedded in the sclera. As soon as the bar was visualized in the sclera, it was removed with the help of Adson forceps. Immediately in the operating room an exploration of the fundus of the left eye was performed, where the corticoid-releasing implant was inside the vitreous and no complications occurred in the retina, and an extra exploration was made 24 hours after and two months later as usual without evidence of any complication (Fig. 1).

Figure 1. Metal bar of the dexamethasone-releasing device.
Hospital Pharmacy was notified the following morning, preventively recalling all similar batches, Abbvie laboratory was notified and an incident occurred in its Pharmacovigilance Department. Abbvie laboratory recalled the applicator involved in the incident at the end of June 2023 from the Hospital Pharmacy Department of the University Hospital of Burgos to analyze the possible cause of the problem. The laboratory’s pharmacovigilance department provided a questionnaire that was completed related to the incident. We have continued injecting the Ozurdex™ implant in our hospital during this summer with other batches and fortunately we are not aware of any similar incident.
Subsequently, this incident was reported to the Board of Directors of the Spanish Society of Retina and Vitreous (SERV) and was also reported to the Pharmacovigilance Area of the Spanish Agency of Medicines and Health Products (AEMPS).
Discussion
Ozurdex injection is a safe and effective procedure to treat CME. In clinical routine, Ozurdex treatment has proven to be a therapy with minimal side effects as described in the ZERO, MEAD and GENEVA studies7.
To our knowledge, this is the first case reported of applicator malfunction of the Ozurdex device related to the mandrel during the injection procedure. It has been reported vitreous haemorrhage after traumatic impact of dexamethasone implant in a vitrectomized eye8.
This rod-shaped implant measures 6 mm in length and 0.46 mm in diameter.
Meyer et al. performed kinematic analysis of the release and velocity of micronized dexamethasone implants using a high-speed camera system5. They concluded that retinal impact energy does not reach damage levels in vitrectomized eyes even if the injection is performed in flat angle. Force testing in basic saline solution (BSS) with dexamethasone implant applicators was 0.024 N with a standard deviation of 0.0318-9. Ernst et al. have shown an ability to cause retinal damage with a force between 0.1 to 0.2N10. But the mandrel probably could cause damage with less force due to heavier than the pellet of micronized dexamethasone implant.
DEX implant propagates at a high speed in vitrectomized BSS and air-filled eyes9. Although our case was also vitrectomized, fortunately in our case the mandrel did not hit the opposite retinal surface.
The actuator bottom releases a folded plastic mechanism that pushes the 2.8 mm long metal rod (mandrel). This stem is fitted but not anchored to a plastic part with a hole of the same cross-section at the end. It is highly probable that in this case, the stem was not firmly inserted into the hole in the plastic piece, so it was released and ended up in the eyeball.
Since the length of the stem (mandrel) is 2.8 mm (Fig. 2) and the distance between the sclerotomy and the contralateral retina varies between 9.22 mm and 18.45 mm in the patient’s eye whose axial length measured with IOLmasterTM (Zeiss) is 23.15 mm (Fig. 3), there is no danger of trauma to the contralateral retina unless the mandrel is propelled with sufficient force and speed and impacts on the contralateral retina. This can occur as in our case due to the mandrel is only held by 0.1 mm which is the portion of the mandrel inside the plastic piece.

Figure 2. Measure of the mandrel introduced in the vitreal cavity. 2.8 mm. 0.1 mm of the mandrel fits inside the plastic piece.
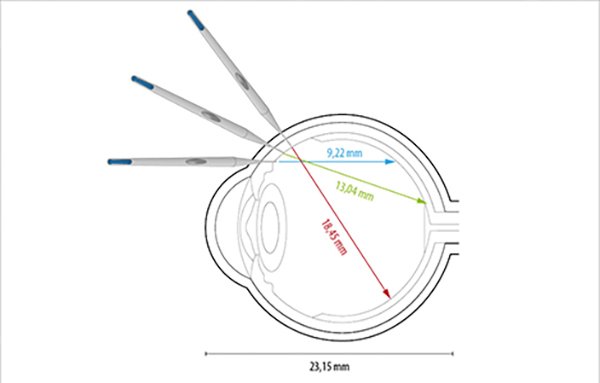
Figure 3. Estimated distance to the contralateral retinal surface based on the angle of the tip of the applicator inserted from sclerotomy.
Conclusion
Although there is no clear scientific evidence establishing the magnitude of energy needed to induce retinal damage after a direct impact, the mandrel of the device could be more damaging than the micronized dexamethasone rod itself. A steep injection angle ensures a longer travel distance, so it may help to reduce the velocity of the implant before reaching the retina. Consideration should also be given to orienting the implant away from the posterior pole.
The retinologist must be aware of this rare non-pharmacological complication in order to prevent it and, should it occur, know how to detect it and provide a quick and correct solution. This rare complication should not be underestimated due to its potential visual threat. In our case, what happened did not lead to a loss of efficacy of the dexamethasone implant. Nor did it have any consequences on the ocular anatomy, so it was not a direct contraindication for a new dose if necessary.
It could be considered to optimize the clamping of the mandrel in the plastic part in a more secure way, not only by pressing it 0.1 mm into the hole of the plastic part to eliminate the possibility of the metal rod entering the vitreous cavity in an uncontrolled manner.
In other hand, it is importatn to emphazise that ophthalmologist may inform and report these complications to the scientific community in order to be aware for future possible incidents.
References