
Figure 1. (A) Small yellowish retinal lesion in the inferior area of the macula outside the fovea (arrow). (B) Retinal hemorrhage in the nasal area of the right eye (arrow).
REVIEW
Ocular inflammatory manifestations induced by dengue virus infection
Marcelo Rudzinskia, b and Adriana E. Echeverríaa, c
a Catedra de Oftalmología, Universidad Católica de las Misiones, Posadas, Misiones, Argentina.
b Consejo Nacional de Investigaciones Científicas y Técnicas, (CONICET), Argentina.
c Hospital de agudos Ramón Madariaga, Parque de la Salud, Posadas, Misiones, Argentina.
Received: July 7th, 2020.
Accepted: July 26th, 2020.
Corresponding author
Dr. Marcelo Rudzinski
Universidad Católica de Misiones
Av. Jauretche 1036
3300 Posadas, Misiones
Argentina
(376) 4469697
rudzinski-investigacion@ucami.edu.ar
Oftalmol Clin Exp (ISSN 1851-2658)
2020; 13(3): 113-126.
Aknowledgements
We would like to thank graphic designer Fabian Goya for helping with the figures.
ABSTRACT
Dengue infection can produce a wide clinical spectrum of inflammatory manifestations in the eye. Ocular manifestations during the critical period of the disease are more frequently associated with vascular damage induced by the viral protein NS1. Sight-threatening retinal conditions in that period include posterior uveitis and dengue maculopathy. Retinal hemorrhages, edema, vasculitis and exudative retinal detachment are the most frequent presentations of posterior uveitis. SD-OCT and OCTA are tools capable to locate the affected retinal layers and capillary plexus involved in the retinopathy. Neuroophthalmological manifestations although infrequent are an important cause of visual disturbance, inducing ophthalmological inter-consults in hospitalized patients. Available treatments include supportive measures, systemic corticosteroids and intravenous immunoglobulin. There is an urgent need for clinical studies to test drugs known to restore vascular permeability as well as new antiviral drug candidates.
Keywords: dengue, ocular manifestations, 2020 epidemic.
Manifestaciones inflamatorias oculares inducidas por el virus del dengue
RESUMEN
La infección por dengue produce un amplio espectro de manifestaciones inflamatorias oculares. Las que ocurren durante el período crítico de la enfermedad están asociadas con daño vascular inducido por la proteína viral NS1. Las manifestaciones retinales que pone en riesgo la visión del paciente durante este período son las uveítis posteriores y la maculopatía por dengue. Hemorragias, edema y vasculitis retinal junto con el desprendimiento exudativo de retina son las manifestaciones oculares más frecuentes de la uveítis posterior. La tomografía de coherencia óptica de dominio espectral (SD-OCT) y la angiografía tomográfica de coherencia óptica (OCTA) son herramientas útiles, capaces de localizar los plexos capilares afectados en esta retinopatía infecciosa. Aunque infrecuentes (respecto del total de pacientes infectados) las manifestaciones neurooftalmológicas son causa de consulta oftalmológica por síntomas visuales e interconsultas de pacientes hospitalizados. Los tratamientos disponibles incluyen medidas de soporte y asistencia general, corticoides sistémicos y tratamiento con inmunoglobulina intravenosa. Existe una urgente necesidad de ensayos clínicos orientados a testear drogas con conocida actividad estabilizadora de la permeabilidad vascular retinal así también como de drogas antivirales.
Palabras clave: dengue, infecciones oculares.
Manifestações inflamatórias oculares induzidas pelo vírus do dengue
RESUMO
A infeção por dengue produz um amplo espectro de manifestações inflamatórias oculares. As que acontecem durante o período crítico da doença estão associadas com dano vascular induzido pela proteína viral NS1. As manifestações retinianas que põem em risco a visão do paciente durante esse período são as uveítes posteriores e a maculopatia por dengue. Hemorragias, edema e vasculite retiniana junto com o desprendimento exudativo de retina são as manifestações oculares mais frequentes da uveíte posterior. A tomografia de coerência óptica de domínio espectral (SD-OCT) e a angiografia tomográfica de coerência óptica (OCTA) são ferramentas úteis, capazes de localizar os plexos capilares afetados nesta retinopatia infeciosa. Embora infrequentes (em relação ao total de pacientes infetados) as manifestações neurooftalmológicas são causa de consulta oftalmológica por sintomas visuais e interconsultas de pacientes hospitalizados. Os tratamentos disponíveis incluem medidas de suporte e assistência geral, corticoides sistêmicos e tratamento com imunoglobulina intravenosa. Existe uma urgente necessidade de ensaios clínicos orientados a testar drogas com conhecida atividade estabilizadora da permeabilidade vascular retiniana como também de drogas antivirais.
Palavras chave: dengue, infeções oculares.
INTRODUCTION
Dengue virus (DENV) belongs to the Flaviviridae virus family. It is an arbovirus, an arthropod borne virus1, meaning that the virus is transmitted by an arthropod.
The mosquito Aedes aegypti is its main vector in urban settings and Aedes albopictus in rural and rainforest areas. The recent expansion of Aedes aegypti distribution in the world resulted in a 30 fold increase in the incidence of dengue disease in the last decades2-3.
DENVs are a group of positive mono-catenary RNA viruses that are antigenically related and grouped according to the human serum response (serotype) in DENV-1 to DENV-4. The genome is an 11 Kb RNA strand with 10 genes coding 3 structural proteins and 7 nonstructural proteins. Protein M and Protein E are the main proteins of the envelope. Protein C forms the capsid. The nonstructural proteins are NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS51.
Dengue is found in tropical and sub-tropical climates worldwide, mostly in urban and semi-urban areas. The global incidence of dengue has grown dramatically in recent decades. There are 100-400 million of estimated infections each year. The largest number of dengue cases ever reported globally was in 2019. The American region alone reported 3.1 million cases, with more than 25,000 classified as severe2. According to the National Health Ministry, Argentina has more than 92.229 dengue suspected cases since week 31 of 2019 (end of July 2019)4. Of those patients, 56492 are confirmed dengue cases. The propagation of the disease from the north of the country during the first trimester of 2020, resulted in the worst known epidemy of dengue in Argentina. Current prevalent serotypes in Argentina are DENV-1 in the northwest, DENV1- and DENV-4 in the center and DENV-1, DEN-2 and DENV-4 in the northeast of the country. The three serotypes (DENV-1, DENV-2 and DENV-4) were also detected in Buenos Aires province4.
Each serotype comprises several genotypes that have specific geographical distributions5. Five genotypes for DENV-1, 6 for DEN-2, 5 for DENV-3 and 4 genotypes for DNV-4. Infection by any of the serotypes generates a humoral immune response. Antibodies providing protection against all serotypes are called heterotypic antibodies and their protection lasts approximately 3 months. Specific antibodies against the infective serotype (homotypic antibodies) provide homotypic protection which was believed to be lifelong6. However, in a recent review of samples from patients in Nicaragua (obtained between epidemic outbreaks 2005 to 2012) homotypic reinfections were found within the same serotype for DENV-1, DENV-2 and DENV-3 serotypes7. Analysis of samples from patients reinfected in a large outbreak in Peru 2010-2011 also showed homotypic reinfection of patients with a DENV-2 American-Asian genotype different from the infecting DENV-2 Asian genotype that primarily infected the patients in 19958. Together the data destroyed the dogma: one-time infection by a homologous DENV serotype.
PATHOPHYSIOLOGY
The DENV NS1 protein is a complex multifunction protein involved in capsid assembly, host immune system evasion and vascular leakage. DENV NS1 has been shown to circulate as a soluble protein at high levels (1-2 μg/ml), correlating with DENV viremia9.
The hallmark of severe dengue is increased vascular permeability. Collapse of the vascular lumen as well as increase endothelial markers were shown in small vessels while no signs of endothelial cell (EC) necrosis or EC DENV infection accompanied the above mentioned changes10. Vessel hyperpermeability was suggested to be either a direct effect of DENV NS1 on the glycocalyx11 or a consequence of the function of several cytokines released by PBMC12 after the activation of Toll-like-receptor-4 TLR413. It was shown that DENV-NS1 can activate sialidases, cathepsin L and heparinases thus triggering the degradation of sialic acid and heparan sulfate at the glycocalyx14. DENV NS1 was shown to induce hyperpermeability in all tested human endothelial cells, with maximal effect on pulmonary endothelial cells15. The vascular leakage is apparently mediated by the specific interaction and subsequent internalization of NS1 in endothelial cells. Recently, the decrease of EC barrier integrity was associated with the activation of the p38MAPK pathway and it could be restored after the treatment with a p38MAPK inhibitor16.
HUMORAL IMMUNE RESPONSE
Antibodies typically protect humans from viruses in 3 ways: A) neutralization (antibody blocks virus interaction with host cell), B) opsonization (antibody coats virus and typically targets it for uptake by macrophages and neutrophils), C) antibody-dependent cellular cytotoxicity (ADCC, where the antibody mediates the destruction of infected cells).
In severe dengue, antibodies play a different detrimental function for the host. Severe dengue most commonly occurs among patients with secondary DENV infections and infants with primary infections. The most widely cited hypothesis for the pathogenesis of severe dengue in a second infection setting is called antibody-dependent enhancement (ADE)17. Human serological studies, as well as animal and in vitro models support the ADE hypothesis. Although the exact mechanisms are not clear, ADE is the process in which DENV complexed with non-neutralizing antibodies can enter into a greater proportion of cells of the mononuclear lineage, such as monocytes, macrophages and dendritic cells, thus increasing the quantity of infected cells and consequently increasing virus production. In dengue, non-neutralizing heterotypic IgG anti-DENV antibodies produced during first DENV infection can form antibody-DENV complexes in the second infection that can allow uptake of DENV by mononuclear cells. DENV then replicates in these macrophages thereby increasing viral production. The uptake of the heterotypic antibody-virion complex occurs after the docking of the immune complex to the Fcγ-R of a mononuclear cell expressed on its surface18. Recently, dengue viral load at presentation and the odds of severe disease were highest among patients with low to intermediate pre-infection antibody titers and lowest among those with the highest antibody titers19.
The role of antibodies against NS1 is still matter of discussion. On the one hand, passive transfer of anti-DENV-NS1 antibodies to mice has proven to avoid lethal encephalitis20. Antibodies that bind sNS1 in circulation were shown to neutralise its vasoactive effects, as demonstrated in a mouse model of NS1-induced vascular leakage12. On the other hand, human antibodies against NS1 may react against human endothelial cells21, platelet antigens22 and anticoagulation factors23. Human antibodies against NS1 can also consequently produce vascular leakage, thrombocytopenia and coagulopathy, possibly as a consequence of NS1 mimicry effect.
CLINICAL SYMPTOMS
In 2009, the WHO changed the definition of dengue infection. Symptomatic dengue can present as undifferentiated fever, dengue and severe dengue. Asymptomatic dengue could represent at least approximately 10% of infected patients24. The main symptom in dengue is fever, usually higher than 38°C, reaching in many patients 40°C and lasting 3-7 days. Myalgia, joint pain, retroocular pain, sore throat and facial erythema could also be present. Dengue can present with alarming signs: abdominal pain, persistent vomiting, clinically evident accumulation, mucosal bleeding, lethargy or restlessness, liver enlargement > 2 cm, increased hemoconcentration concurrent with platelet decline25. Alarming signs indicate the need for patient hospitalization to avoid severe complications. Severe dengue can present as severe plasma leakage, severe bleeding or severe organ involvement25.
After the mosquito bite there is a period of up to 7 days of incubation without clinical symptoms. The febrile period lasts between 4-7 days and is followed by the critical period. This period lasts approximately 2 days and is characterized by defervescence (decrease in body temperature), hemoconcentration and decrease of platelet count. The recovery phase is the last period and is characterized by the return of plasma to the vasculature with near normal hematocrit values.
DIAGNOSIS OF DENGUE INFECTION
Approximately 24 to 48 h before the febrile period there is already detectable viremia which lasts till the start of the critical period. During viremia either RNA or antigen detection methods could be used for the diagnosis of dengue infection. The CDC and the WHO recommend RT-PCR for the diagnosis of dengue disease, during this period. After the febrile period, the diagnosis is based on the detection of specific IgM and IgG antibodies1, 25.
Ocular manifestations of dengue
Anterior manifestations
Subconjunctival hemorrhages are the most frequent ocular sign reported by patients with dengue infection. Up to 37,3% of patients with thrombocytopenia presented it during the critical period26. Diffuse epitelial keratitis as well as stromal keratitis has been reported in patients with acute dengue infection27. Necrotizing scleritis was observed in a Japanese patient without precedent autoimmune diseases who became infected with dengue virus during holidays in the Philippines28. The patient’s scleritis was controlled with methylprednisolone pulse-therapy. There were no further recurrences after treatment, but the patient developed scleral thinning over the following years.
Other anterior manifestation of dengue infection is acute angle closure glaucoma (AAG). AAG associated with dengue can present with unilateral or bilateral involvement29. This entity may be associated with extensive choroidal effusions30 or as a consequence of an iris plateau configuration31.
Patients presenting with ocular pain, eye redness and photophobia few weeks or months after acute dengue fever are frequently diagnosed with presumed dengue associated anterior uveitis32. Anterior uveitis (AU) is less frequently diagnosed during acute disease33. AU can present as unilateral or bilateral uveitis, more frequently as non-granulomatous uveitis34. The etiological mechanism seems to be an autoimmune reaction following dengue infection since the patients respond to topical or periocular corticosteroids.
Posterior manifestations
Intermediate uveitis is an infrequent presentation of dengue infection. In a series of 65 eyes of patients with visual complains associated with dengue infection only 8 had intermediate uveitis35. Approximately 11% of patients who experience dengue maculopathy, a frequent clinical presentation of dengue infection, also have signs of intermediate uveitis36.
Posterior uveitis (PU) is a common complication of dengue infection. PU by dengue is one of the three more frequent aetiologies of posterior uveitis in Singapore37. Clinical presentations of PU in dengue comprise vascular retinitis, exudative retinal detachment, chorioretinitis and neuroretinitis (Table 1). In a study that included 41 patients, 15 of 65 eyes with posterior ocular manifestations had retinal vasculitis38. Other retinal signs associated with retinal vasculitis are intraretinal hemorrhages and exudative retinal detachment39-40. Exudative retinal detachment was observed in 13 of those 15 previously mentioned eyes with severe retinal vasculitis in the study published by Theo SC. In severe cases of retinal vasculitis retinal ischemia may be present due to microvascular occlusion41. When the macular retinal detachment has fibrinous material a pseudophypopion can be observed as that reported in two patients from Malaysia42.
Multifocal areas of chorioretinitis with retinal vasculitis, retinal hemorrhages and exudates were described by Tabbara K in 2 patients from Saudi Arabia43.The patients had leukopenia and thrombocytopenia with high titers of IgM anti-dengue. The healing of the lesions left discrete atrophic chorioretinal scars with nummular shapes. Chorioretinal lesions during dengue infection may display features in the spectrum of acute posterior multifocal placoid pigment epitheliopathy (APMPPE)44 or choroiditis45. Multifocal chorioretinal lesions resembling APMPPE were described in a patient who developed dengue fever after visiting the Caribbean islands. The OCT revealed disruption of the ellipsoid layers and RPE. The healing of the lesion left discrete chorioretinal scars44. Severe decrease in visual acuity and persistent scotoma in patients with dengue infection were associated with disruption of the outer neurosensory retina involving the outer limiting membrane, the myoid and ellipsoid zone as well as the outer segments of the photoreceptors, findings similar to those described in acute zonal occult outer retinopathy, AZOOR46-47.
Concomitant inflammation of the optic nerve and macula can manifest as neuroretinitis. A classical presentation of neuroretinitis with vitritis, papillitis, exudates forming a macular star was reported in a Brazilian patient with acute dengue fever48.
Two reports of blinding panophthalmitis in patients with severe dengue were recently described49-50. In both cases, the patients were admitted to hospital. They developed panophthalmitis due to secondary bacterial infection (Staphylococcus epidermidis and Bacillus Cereus). Both patients survived but the infected eyes were eviscerated.
Table 1. Uveitis caused by dengue virus infection. Clinical presentations reported frequencies and response to treatment.
Uveitis |
Clinical presentation |
Frequency |
Response to treatment |
Anterior |
Unilateral or bilateral |
Frequent after the recovery period, up to 5 months |
Good response to topical corticosteroids |
Intermediate |
Associated with dengue maculopathy |
Rare |
Insufficient data |
Posterior |
Retinal vasculitis |
Frequent |
Good response to systemic corticosteroids |
Exudative retinal detachment |
Frequent |
||
Multifocal chorioretinitis |
Less frequent |
||
Acute posterior multifocal placoid pigment epitheliopathy (APMPPE) |
|||
Choroiditis |
|||
Acute zonal occult outer retinopathy (AZOOR) |
|||
Neuroretinitis (macular star) |
Rare |
Clinical spectrum of dengue maculopathy
Dengue maculopathy is the most frequent cause of visual complains in patients with dengue infection. Approximately 10% of infected patients admitted to hospitals will develop dengue maculopathy (DM)51 or complain of blurry vision52. Main visual symptoms are blurry vision and or scotomata51. Decrease of visual acuity (VA) typically appears during the critical period, but it can also present up to 30 days after the febrile period. Other less frequent symptoms are myodesopsia and metamorphopsia. The decrease in VA at the time of diagnosis is mild to moderate in most patients. Approximately 69% of patients had a VA of 20/200 or better according to Teoh et al35. Bilateral involvement is very frequent but usually asymmetrical35-36. The triad of photopsia, myodesopsia and blurry vision was highly predictive of retinal hemorrhages52.
According to its pathogenesis and ordered by frequency: macular edema, macular retinal detachment + severe vasculitis, macular hemorrhage and foveolitis are the most frequent clinical presentations of DM42. In a small case series, Chan et al found that macular hemorrhages were the most frequent cause of decreased visual acuity followed by retinal vasculitis and macular retinal detachment33.
Three different patterns were described in DM using standard optical coherence tomography (OCT): diffuse retinal edema, cystoid retinal edema and foveolitis (Table 2). The latter is characterized by a thickening and hyperreflectivity of the subfoveal outer retinal layers38.
The use of OCT angiography (OCTA) in patients with foveolitis and outer maculopathy shows flow deficit of the superficial retinal capillary plexus in 43,75% of the patients. Areas with flow deficit in the deep retinal capillary plexus were present in all patients. None of the eyes showed presence of choriocapillaris flow deficit areas53.
Recently a new clinical presentation involving the macular neuroretina during dengue disease was described. Acute macular neuroretinopathy (AMN) is characterized by ischemia of the retinal deep capillary plexus (DCP)54. Clinically, retinal exudation can present around the foveal area. Macular retinopathy can be accompanied by vitritis and by optic nerve inflammation. Fluorescein angiography may show vascular leakage in the macular area and optic disc staining in the late phase. The OCTA displays involvement of the different vascular retinal plexus but only the deep retinal capillary plexus are associated with the disruption of the IS/OS junction of the retina, a typical finding in optical coherence tomography (OCT) of AMN patients55.
Other reported immediately after macular complication dengue infection is choroidal neovascularization56. Veloso et al described the case of a 54-year-old female patient complaining of decreased visual acuity in her left eye two weeks after dengue fever. A diagnosis of classic CNV was made with FA and confirmed with SD-OCT. The patient was treated with ranibizumab intravitreal injections reaching a BCVA of 20/20 after the treatment.
Table 2. Dengue maculopathy: clinical presentations and findings on fluorescein angiography (FA), optical coherence tomography (OCT) and OCT-angiography (OCTA).
Dengue maculopathy |
Clinical presentation |
Fluorescein-angiography (FA) |
OCT pattern |
OCT-A pattern |
Macular edema |
Diffuse macular edema |
Late hyper-fluorescence. Diffuse fluorescein leakage |
Diffuse retinal thickening (DRT), around central/ paracentral fovea. Loss of foveal dimple |
Flow deficit in the superficial capillary plexus |
Cystoid macular edema |
Hyper-fluorescence due to leakage in cystoid retinal spaces in middle and late stages |
Cystoid fluid spaces in the middle layers of the retina |
||
Exudative retinal detachment (ERD) + severe vasculitis |
ERD + vasculitis |
Perifoveal fluorescein leakage and hyper-fluorescence in middle and late stages |
Subretinal fluid, cystoid macular edema may also be present |
Not described for dengue |
Macular hemorrhage |
Macular hemorrhage |
Hypo-fluorescence due to fluorescence blocking in the macular area |
Accumulation of fluid of medium reflectivity at the retina affecting the normal layers architecture |
Not described for dengue |
Foveolitis |
White yellowish dots in the fovea |
Perivascular foveal leakage and blockage of arteriolar fluorescence |
Hyper-reflectivity at the outer plexiform and outer nuclear layers |
Flow deficit in the superficial and deep capillary plexus |
Acute macular neuroretinopathy (AMN) |
Exhudation around vessels of macular area |
Fluorescein vascular leakage around the macular vessels. Optic disc staining in late phase |
Disruption of the IS/OS junction in the outer retina |
Flow deficit in the 3 capillary plexus, low VA is associated with flow deficit of the deep capillary plexus |
Neuro-ophthalmological manifestations
Approximately 97% of patients with dengue fever will complain of headaches57. Neurological signs upon dengue infection are reported in only 1% to 5% of patients58 and neuro-ophthalmic manifestations are rare or infrequent59. Nevertheless, in the clinical history of 2 out of the 3 patients described below, who sought ophthalmic consultation during the 2020 epidemic in Misiones, the primary ocular manifestation was neuro ophthalmological. Encephalitis and dengue encephalopathy are the most frequent neurological manifestations, followed by Guillain-Barré syndrome and nerve palsies. Abducens nerve palsies (VI nerve palsy) is a frequent form of nerve palsies associated with dengue infection. Abducens palsies as well as the above mentioned neurological manifestations are more frequent during the critical period60-62. Optic neuritis was reported to occur in 0.1% to 1.5% of patients with dengue infection35. It could present as either inflammation of the optic disc or as retrobulbar optic neuritis63-64. Recently, Lana-Peixoto et al reported two cases of neuromyelitis optica spectrum disorder (NMOSD) in patients positive for serum AQP4-antibody suggesting that dengue infection may trigger seropositive NMOSD65.
TREATMENT
Good resolution of anterior mild inflammatory manifestations may be achieved with topical corticosteroids such as in mild to moderate anterior uveitis66. Local periocular treatment (sub-Tenon’s triamcinolone injection) can be used in severe forms of unilateral anterior uveitis and mild cases of dengue retinopathy. The use of intravitreal triamcinolone can be considered in patients with unilateral maculopathy36.
Systemics corticosteroids are needed in severe scleritis and in posterior manifestations where immune mediated mechanisms are suspected (retinal vasculitis, AMN and foveolitis). In patients whose visual acuity was lower than 20/100, methylprednisolone pulse therapy (1 g/day) was efficient in the treatment of severe posterior uveitis and optic neuritis. Pulse therapy should be followed by oral prednisone with slow tapering.
There are few reports of severe PU and dengue maculopathy patients with VA lower than 0.1 treated with intravenous Immunoglobulin (0,4 g/kg/ day) for 3 days. Visual acuity was restored to 0.5 after 15 days of treatment67.
Despite the fact that anti-VEGF therapy had shown to restore vascular permeability in macular edema associated with retinal vasculopathy, there are no reports regarding its use in dengue maculopathy except for choroidal neovascularization56. The use of such well-known agents could be hypothetically useful, specially in macular edema caused by dengue infection.
OCULAR FINDINGS IN PATIENTS EXAMINED DURING THE 2020 DENGUE EPIDEMIC
Case 1 (retroorbital pain-normal vision)
A 42 years old man seeks consultation due to retroocular pain. He has been diagnosed dengue fever by a medical doctor specialized in tropical medicine. He suffered high fever, asthenia and myalgia for 5 days. He is in the recovery phase but still complains of bilateral retroocular pain. A diagnosis of dengue is suspected based on the symptoms and a positive antigen NS1 ELISA test. His best corrected visual acuity (BCVA) was 20/20 in both eyes. The patient had normal pupillary reflexes and ocular movements. There were no anterior signs of ocular inflammation. Intraocular pressure was 16 mmHg in both eyes. There were scattered retinal hemorrhages outside the posterior pole in both eyes (Fig. 1). A small, yellow, well-defined deep retinal dot like those described in foveolitis was observed in the superior part of the macula, outside the fovea, in the right eye (Fig. 1). The patient received no treatment and was controlled 15 days later. Dengue disease was confirmed by the presence of anti DENV positive IgM and IgG results. Resolution of the hemorrhages and the macular spot was confirmed after 3 months of the initial examination.

Figure 1. (A) Small yellowish retinal lesion in the inferior area of the macula outside the fovea (arrow). (B) Retinal hemorrhage in the nasal area of the right eye (arrow).
Case 2 (severe unilateral decrease in visual acuity)
A 38-year-old male patient seeks ophthalmic consultation as an outpatient in a private clinic. He complains of decrease in visual acuity in the left eye for the last 3 days accompanied by pain when moving the eye. Three weeks before he had 4-day episode of fever, malaise and headaches. He also referred a history of relatives with dengue infection. BCVA was 20/20 in the right eye and counting fingers on the left eye. There was a RAPD in the left eye and severe decrease in the chromatic vision. The anterior biomicroscopy was normal as well as the intraocular pressure in both eyes. The fundus of the eye revealed a left optic disc with diffuse borders and inflammation (Fig. 2). There were no signs of retinopathy. The right eye fundus was normal. Routine lab exams (hematocrit, ESD, kidney and liver function assays) as well as an MRI with gadolinium of the CNS and serology for dengue, syphilis and toxoplasma gondii were ordered. The patient did not want to receive corticosteroid pulsetherapy in a hospital, due to the COVID-19 pandemic, so he started oral meprednisone 1mg/kg/day as an outpatient. The CNS MRI results, and the routine lab exams were normal. VDRL and serology for syphilis were negative. A positive IgM and IgG anti-DENV was detected. Serology for toxoplasma gondii indicated a chronic infection, a regular finding in an adult patient from Misiones. The patient visual acuity started to improve daily. After 4 weeks of treatment the patient attained 20/20 vision on the left eye and his visual fields were normal.

Figure 2. Images of the right and left posterior fundus of the eye. The right optic disc and macular area appears normal. The left optic disc has diffused borders with exudative changes that together with the clinical presentation of the patient led to the diagnosis of left optic neuritis.
Case 3 (abducens nerve palsies)
A 42 years old man seek consultation at the emergency room. He complained of dysesthesia in his lower extremities, dysarthria and diplopia for the last 48 h. He had fever, myalgia and gastrointestinal symptoms for the last 10 days. He explains that due to the SARS-Cov2 pandemic intercurrence he received telephonic assessment and was prescribed paracetamol. The clinician that examined him in the emergency room ordered routine blood laboratory test (hematocrit, WBC count, ESR, PCR, liver and kidney function laboratory tests), a Central Nervous System CAT-scan without gadolinium and an ophthalmologic examination. The CNS CAT-scan was normal. The ophthalmic examination revealed: BCVA of 20/20 in both eyes and a 30-degree esotropia in the left eye. Limited abduction of the left eye was confirmed and a left abducens nerve palsy was diagnosed. Due to his poor general health status (fever, tremors and asthenia) the patient was admitted to the hospital. A fundus examination showed performed revealing retinal exudates along the temporal vessel in the right eye and a small macular hemorrhage in the left eye. The neurologists that examined the patient ordered a CNS MRI with gadolinium and an angio-MRI. The exams revealed an acute ischemic event at the knee of the corpus callosum and also in the left cerebellum (Fig. 3). Due to the patient’s signs and symptoms and the SARS-Cov-2 pandemic + dengue epidemic intercurrence, the patient was isolated and nasopharyngeal and blood samples were obtained. Seventy-two hours later the SARS-Cov-2 PCR result was negative and the NS1 for dengue was positive. No specific treatment was administered during the days of hospital admission. The neurological symptoms and ocular motor paresis improved without specific treatment. The diagnosis of dengue disease was confirmed with IgM and IgG positive serology. A diagnosis of reversible splenial syndrome (RESLES) was made based on signs and symptoms manifested by the patient, MRI images and the evolution of the patient. Although RESLES was recently described in a patient with dengue infection, this is the first case were RESLES is described with angiopathic changes in the retina.
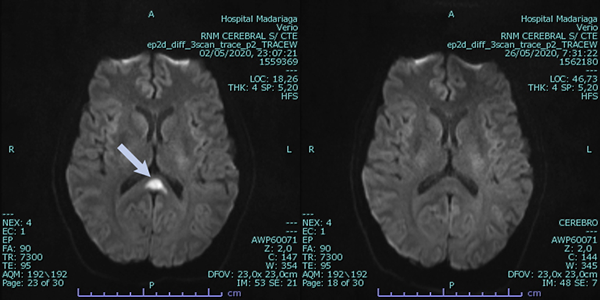
Figure 3. Central Nervous System MRI with gadolinium showing lesion in the central splenium of the corpus callosum in the diffusion-weighted imaging (image on the right). After two weeks the lesion in the corpus callosum disappeared (image on the left).
DISCUSSION
The ocular clinical spectrum caused by dengue infection is not random. It is influenced by the degree of vascular endothelial damage induced by the virus as well as the inflammation induced by the immune response of the host. A predominance of retinal hemorrhages and perivascular exudates in the fundus examination are an indicative of endothelial cell damage, the presence of retinal vasculitis and optic neuritis are more likely associated with a significative humoral immune response. Mechanistically it seems that early in the evolution of the disease (symptomatic and critical period), ocular and neuro-ophthalmological manifestations may be related to direct viral effects, immune mediated or due to systemic or metabolic complications (thrombocytopenia, leukopenia or hypoalbuminemia), while post-recovery and late manifestations are mainly immune mediated.
The posterior exudative and hemorrhagic manifestations are also influenced by the depth of the retinal capillary plexus disrupted. The more superficial the retinal capillary plexus affected the more superficial the exudation in the retina, resembling Purtscher-like retinopathy68. On the other hand, posterior deep manifestations such as foveolitis or AMN indicate the involvement of deep retinal capillary plexus. Knowledge of the retinal plexus involved is important because the alteration of deep retinal plexus is associated with disruption of the ellipsoid and interdigitation zone of the outer retina and a consequent persistent decreased visual acuity and scotoma as described in AMN55. Together, the results indicate a relevant role of OCTA in the diagnosis of the retinopathy upon dengue infection.
Endothelial damage and vascular leakage are the main pathogenetic mechanism during the first two weeks of the disease while immunological mechanism may persist for few months. After an extensive search in the online clinical databases only one publication of chronic uveitis was associated with dengue infection. Recently, however, in a population based cohort study dengue infection was associated with the development of a higher frequency of autoimmune diseases. The list of diseases included known etiology for anterior uveitis (Reiter’s syndrome) and posterior or diffuse uveitis (systemic vasculitis) as well as optic neuritis (multiple sclerosis)69.
Despite our limited experience with the disease, the review of dengue epidemics, including the 2020 dengue epidemic in Misiones, taught us valuable lessons: 1) patients with dengue infection may be visually asymptomatic but still present retinal changes, while the disease is not affecting the fovea. Also, afebrile or asymptomatic dengue infected patients may develop exudative or inflammatory ocular manifestations, so dengue infection should be ruled-out if clinical ocular signs are compatible with dengue diagnosis. 2) The use of new technologies such as SD-OCT and OCTA can help in the diagnosis of the retinopathy and follow up of patients. 3) Neuro-ophthalmological signs may not be that infrequent as described. Two out of the 3 patients that seek ophthalmologic examination and were examined by the authors, had neuro-ophthalmological manifestations. 4) Immune mediated ocular manifestations such as anterior uveitis, retinal vasculitis and optic neuritis may appear few weeks or months later after the critical period of the disease. 5) The treatment of posterior uveitis and dengue maculopathy has been limited to corticosteroids and intravenous immunoglobulin. Clinical studies using agents known to restore vascular permeability such as bevacizumab, ranibizumab and aflibercept can be projected to evaluate its clinical use in dengue maculopathy as additional treatments.
REFERENCES
1. Carballal G, Oubiña JR. Virología médica. Buenos Aires: Corpus, 2015.
2. World Health Organization. Dengue and severe dengue [online]. Geneve: WHO, June 2020 (Fact sheets). Available: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
3. Guzman MG, Gubler DJ, Izquierdo A et al. Dengue infection. Nature Rev Dis Primers 2016; 2: 16055.
4. Argentina. Ministerio de Salud. Dirección Nacional de Epidemiología e Información Estratégica. Vigilancia de dengue y otros arbovirus. Boletín Integrado de Vigilancia Epidemiológica 2020; 502: 6-19.
5. Acosta EG, Bartenschlager R. Paradoxical role of antibodies in dengue virus infections: considerations for prophylactic vaccine development. Expert Rev Vaccines 2016; 15: 467-482.
6. Wilken L, Rimmelzwaan GF. Adaptative immunity to dengue virus: slippery slope or solid ground for rational vaccine design? Pathogens 2020; 9: 470.
7. Waggoner JJ, Balmaseda A, Gresh L et al. Homotypic dengue virus reinfections in Nicaraguan children. J Infect Dis 2016; 214: 986-993.
8. Forshey BM, Stoddard ST, Morrison AC. Dengue viruses and lifelong immunity: reevaluating the conventional wisdom. J Infect Dis 2016; 214: 979-981.
9. Libraty DH, Young PR, Pickering D et al. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis 2002; 186: 1165-1168.
10. Sahaphong S, Riengrojpitak S, Bhamarapravati N, Chirachariyavej T. Electron microscopic study of the vascular endothelial cell in dengue hemorrhagic fever. Southeast Asian J Trop Med Public Health 1980; 11: 194-204.
11. Liao B, Tang Y, Hu F et al. Serum levels of soluble vascular cell adhesion molecules may correlate with the severity of dengue virus-1 infection in adults. Emerg Microbes Infect 2015; 4: e24.
12. Beatty PR, Puerta-Guardo H, Killingbeck SS et al. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci Transl Med 2015; 7: 304: 304ra141.
13. Modhiran N, Watterson D, Muller DA et al. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci Transl Med 2015; 7: 304ra142.
14. Puerta-Guardo H, Glasner DR, Harris E. Dengue virus NS1 disrupts the endothelial glycocalyx, leading to hyperpermeability. PLoS Pathog 2016; 12: e1005738.
15. Puerta-Guardo H, Glasner DR, Espinosa DA et al. Flavivirus NS1 triggers tissue-specific vascular endothelial dysfunction reflecting disease tropism. Cell Rep-2019; 26: 1598-1613.e8.
16. Barbachano-Guerrero A, Endy TP, King CA et al. Dengue virus non-structural protein 1 activates the p38 MAPK pathway to decrease barrier integrity in primary human endothelial cells. J Gen Virol 2020; 101: 484-496.
17. Guzman MG, Alvarez M, Halstead SB. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch Virol 2013; 158: 1445-1459.
18. Katzelnick LC, Gresh L, Halloran ME et al. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017; 358: 929-932.
19. Waggoner JJ, Katzelnick LC, Burger-Calderon R et al. Antibody-dependent enhancement of severe disease is mediated by serum viral load in pediatric dengue virus infections. J Infect Dis 2020; 221: 1846-1854.
20. Henchal EA, Henchal LS, Schlesinger JJ et al. Synergistic interactions of anti-NS1 monoclonal antibodies protect passively immunized mice from lethal challenge with dengue 2 virus. J Gen Virol 1988; 69: 2101-2107.
21. Falconar AK. The dengue virus nonstructural-1 protein (NS1) generates antibodies to common epitopes on human blood clotting, integrin/adhesin proteins and binds to human endothelial cells: potential implications in haemorrhagic fever pathogenesis. Arch Virol 1997; 142: 897-916.
22. Wan SW, Yang YW, Chu YT et al. Anti-dengue virus nonstructural protein 1 antibodies contribute to platelet phagocytosis by macrophages. Thromb Haemost 2016; 115: 646-656.
23. Chuang YC, Lin YS, Liu HS, Yeh TM. Molecular mimicry between dengue virus and coagulation factors induces antibodies to inhibit thrombin activity and enhance fibrinolysis. J Virol 2014; 88: 13759-13768.
24. Tan W, Liew JWK, Selvarajoo S et al. Inapparent dengue in a community living among dengue-positive Aedes mosquitoes and in a hospital in Klang Valley, Malaysia. Acta Trop 2020; 204: 105330.
25. Centers for Disease Control and Prevention. Dengue: with or without warning signs [online]. Atlanta: Centers for Disease Control and Prevention. Available: https://www.cdc.gov/dengue/training/cme/ccm/page47831.html
26. Kapoor HK, Bhai S, Jhon M, Xavier J. Ocular manifestations of dengue fever in an East Indian epidemic. Can J Ophthalmol 2006; 41: 741-746.
27. Chhavi N, Venkatesh C, Soundararajan P, Gunasekaran D. Unusual ocular manifestations of dengue fever in a young girl. Indian J Pediatr 2013; 80: 522-523.
28. Kamoi K, Mochizuki M, Ohno-Matsui K. Dengue fever-associated necrotizing scleritis: a case report with long-term follow-up. Medicine (Baltimore) 2018; 32: e11875.
29. Pierre Filho JTP, Carvalho Filho JP, Pierre ETL. Bilateral acute angle closure glaucoma in a patient with dengue fever: case report. Arq Bras Oftalmol 2008; 71: 265-268.
30. Saranappa SBS, Sowbhagya HN. Panophthalmitis in dengue fever. Indian Pediatr 2012; 49: 760.
31. Levaggi ND, Lucas AN, Barletta JAE. Bilateral acute angle closure in a patient with dengue fever: a case report. Arq Bras Oftalmol 2017; 80: 266-267.
32. Antlanger M, Shaw SJ, Kurup SK. Presumed dengue-associated immune-mediated uveitis. Can J Ophthalmol 2011; 46: 92-93.
33. Chan DPL, Teoh SCB, Tan CDH et al. Eye Institute Dengue-Related Ophthalmic Complications Workgroup. Ophthalmic complications of dengue. Emerg Infect Dis 2006; 12: 285-289.
34. Gupta A, Srinivasan R, Setia S et al. Uveitis following dengue fever. Eye (Lond) 2009; 23: 873-876.
35. Teoh SC, Chan DP, Nah GK et al. A re-look at ocular complications in dengue fever and dengue haemorrhagic fever. Dengue Bull 2006; 30: 184-190.
36. Bacsal KE, Chee SP, Cheng CL, Flores JVP. Dengue-associated maculopathy. Arch Ophthalmol 2007; 125: 501-510.
37. Siak J, Jansen A, Waduthantri S et al. The pattern of uveitis among Chinese, Malays, and Indians in Singapore. Ocul Immunol Inflamm 2017; 25: S81-S93.
38. Teoh SC, Chee CK, Laude A et al. Eye Institute Dengue-related Ophthalmic Complications Workgroup. Optical coherence tomography patterns as predictors of visual outcome in dengue-related maculopathy. Retina 2010; 30: 390-398.
39. Siqueira RC, Vitral NP, Campos WR et al. Ocular manifestations in dengue fever. Ocul Immunol Inflamm 2002; 12: 323-327.
40. Lim WK, Mathur R, Koh A et al. Ocular manifestations of dengue fever. Ophthalmology 2004; 111: 2057-2064.
41. Koundanya VV, Chowdhary N, Agarwal M, Katre P. Secondary dengue retinitis with associated occlusive retinal vasculitis. J Ophthalmic Inflamm Infect 2019; 9: 7.
42. Ng CWK, Tai PY, Mohamed SO. Dengue maculopathy associated with choroidopathy and pseudohypopyon: a case series. Ocul Immunol Inflamm 2018; 26: 666-670.
43. Tabbara K. Dengue retinochoroiditis. Ann Saudi Med 2012; 32: 530-533.
44. Goldhart R, Patel H, Davis JL. Acute posterior multifocal placoid pigment epitheliopathy following dengue fever: a new association for an old disease. Ocul Immunol Inflamm 2016; 24: 610-614.
45. Yadav HM, Majumder PD, Biswas J. Dengue associated choroiditis: a rare entity. J Ophthalmic Inflamm Infect 2017; 7: 14.
46. Fang PP, Pfau M, Holz FG, Finger RP. Persistent visual loss in dengue fever due to outer retinal damage. Clin Exp Ophthalmol 2017; 45: 747-749.
47. Oliver GF, Carr JM, Smith JR. Emerging infectious uveitis: Chikungunya, dengue, Zika and Ebola: A review. Clin Exp Ophthalmol 2019; 47: 372-380.
48. de Amorim Garcia CA, Gomes AHB, de Oliveira AGF. Bilateral stellar neuroretinitis in a patient with dengue fever. Eye (Lond) 2006; 20: 1382-1383.
49. Arya D, Das S, Shah G, Gandhi A. Panophthalmitis associated with scleral necrosis in dengue hemorrhagic fever. Indian J Ophthalmol 2019; 67: 1775-1777.
50. Kamal R, Shah D, Sharma S et al. Culture-positive unilateral panophthalmitis in a serology-positive case of dengue hemorrhagic fever. Indian J Ophthalmol 2018; 66: 1017-1019.
51. Su DHW, Bacsal K, Chee SP et al. Dengue Maculopathy Study Group. Prevalence of dengue maculopathy in patients hospitalized for dengue fever. Ophthalmology 2007; 114: 1743-1747.
52. Seet RCS, Quek AML, Lim ECH. Symptoms and risk factors of ocular complications following dengue infection. J Clin Virol 2007; 38: 101-105.
53. Agarwal A, Aggarwal K, Dogra M et al. OCTA Study Group. Dengue-induced inflammatory, ischemic foveolitis and outer maculopathy: a swept-source imaging evaluation. Ophthalmol Retina 2019; 3: 170-177.
54. Li M, Zhang X, Ji Y et al. Acute macular neuroretinopathy in dengue fever: short-term prospectively followed up case series. JAMA Ophthalmol 2015; 133: 1329-1333.
55. Aggarwal K, Agarwal A, Katoch D et al. Optical coherence tomography angiography features of acute macular neuroretinopathy in dengue fever. Indian J Ophthalmol 2017; 65: 1235-1238.
56. Veloso CE, Schmidt-Erfurth U, Nehemy MB. Choroidal neovascularization induced by immunogenic alteration of the retinal pigment epithelium in dengue fever. Case Rep Ophthalmol 2015; 6: 18-23.
57. Domingues RB, Kuster GW, Onuki de Castro FL et al. Headache features in patients with dengue virus infection. Cephalalgia 2006; 26: 879-882.
58. Carod-Artal FJ, Wichmann O, Farrar J, Gascón J. Neurological complications of dengue virus infection. Lancet Nuerol 2013; 12: 906-919.
59. Somkijrungroj T, Kongwattananon W. Ocular manifestations of dengue. Curr Opin Ophthalmol 2019; 30: 500-505.
60. Shivanthan MC, Ratnayake EC, Wijesiriwardena BC et al. Paralytic squint due to abducens nerve palsy: a rare consequence of dengue fever. BMC Infect Dis 2012; 16: 156.
61. Mishra A, Shukla S, Aggarwal S, Chaudhary B. Lateral rectus palsy in a case of dengue fever. Med J Armed Forces India 2015; 71 (suppl.1): S101-S103.
62. Mazliha M, Boo YL, Chin PW. Isolated unilateral sixth cranial nerve palsy: a rare presentation of dengue fever. Malays Fam Physician 2016; 11: 25-26.
63. Preechawat P, Poonyathalang A. Bilateral optic neuritis after dengue viral infection. J Neuroophthalmol 2005; 25: 51-52.
64. Boo YL, Lim SY, Chin PW, Hoo FK. Bilateral optic neuritis with maculopathy: a rare manifestation of dengue fever. Malays Fam Physician 2017; 12: 32-34.
65. Lana-Peixoto MA, Pedrosa D, Talim N et al. Neuromyelitis optica spectrum disorder associated with dengue virus infection. J Neuroimmunol 2018; 318: 53-55.
66. Ng AW, Teoh SC. Dengue eye diseases. Surv Ophthalmol 2015; 60: 106-114.
67. Chang PEJ, Cheng CL, Asok K et al. Visual disturbances in dengue fever: an answer at last? Singapore Med J 2007; 48: e71-e73.
68. Lima LH, Vianello S, Pimentel S et al. Dengue fever presenting as purtscher-like retinopathy. Ocul Immunol Inflamm 2018; 26: 660-665.
69. Li HM, Huang YK, Su YC, Kao CH. Increased risk of autoimmune diseases in dengue patients: a population-based cohort study. J Infect 2018; 77: 212-219.