IMÁGENES CIENTÍFICAS
Suspended scattering particles in motion
Juan Manuel López
Department of Ophthalmology, University of Paris Est-Creteil, Créteil, France.
Department of Ophthalmology, Centre Hospitalier Intercommunal de Créteil, Créteil, France.
Received: August 28th, 2022.
Aproved: October 18th, 2022.
Corresponding author
Dr. Juan Manuel López
Centre Hospitalier Intercommunal de Créteil
Department of Ophthalmology
40, avenue de Verdun
Créteil, 94100, France
drlopezjuan@gmail.com
Oftalmol Clin Exp (ISSNe 1851-2658)
2022; 15(4): e501-e504.
Funding
The author receives support from IOFF e.V and my sponsor. I agree to allow the IOFF e.V to publish the paper on a platform to make it accessible for IOFF e.V and others.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Suspended scattering particles in motion (SSPiM) represent actual flow of suspended particules in intraretinal fluid. This hyperreflective fluid corresponding to SSPiM has side described more frequently in Henle’s fiber layer (HFL) than the inner nuclear layer (INL) and was highly associated with hyperreflective material (HRM) found bordering the fluid. SSPiM and corresponding hyperreflective intraretinal fluid are seen in various exudative maculopathies.
A 67 year-old-male presented with moderate non proliferative diabetic retinopathy (NPDR) and treatment-naive diabetic macular edema (DME) in his right eye.
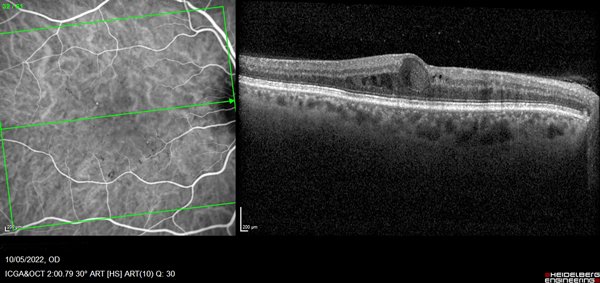
Optical coherence tomography (OCT Spectralis® HRA, Heidelberg Engineering Inc, Germany) scan showed intraretinal oval accumulations of fluid with increased OCT signal intensity corresponded to regions of SSPiM structures in the outer nuclear layer, adjacent to the outer plexiform layer. Cross-sectional flow signal overlaid OCT scans showed that these structures had highly prominent flow signals that were clearly different from projection artifact signals originating in the vasculature of inner retinal layers (Fig. 1).

Figure 1. OCT showed in right eye an intraretinal cyst with hyperreflective fluid in HFL and is bordered by hyperreflective materials.
Optical coherence tomography angiography (SS-OCTA, PlexElite™, Carl Zeiss Meditec) en face revealed star-shaped flow signals in the avascular outer retinal slab. Signal patterns were identical to those associated with the accumulations seen in structural en face OCT. These areas of hyperreflective intreretinal fluid (IRF) tend to localize around the foveal avascular zone (FAZ) and within the deep retinal plexus. SSPiM always is presented at the edge or border of a vascular plexus at a vascular-avascular junction (Fig. 2).
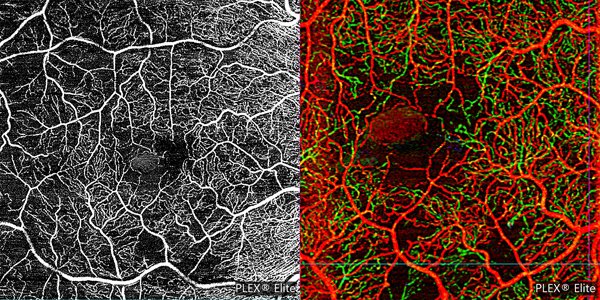
Figura 2. On eye right the OCTA (A) 6 mm x 6 mm and (B) 3 mm × 3 mm depth-encoded color showed the SSPiM on the en face image corresponding to the hyperreflective fluid begins at the border of the FAZ and extends peripherally. On depth-encoded images red corresponds to the superficial retinal layer, and green to the deep retinal layer.
These areas of hyperreflective fluid are generally found in the outer nuclear layer, adjacent to the outer plexiform layer, and are anatomically associated with hyperreflective material (HRM), particularly in the outer retinal layers. Kashani et al was looking at single cross-sections of SSPiM in 76 cases (60 eyes with diabetic retinopathy, 9 eyes with retinal vein oclusion, and 5 eyes neovascular age-related macular degeneration, 1 eye with macroaneurysm, and 1 eye with radiation retinopathy), 88.2% displayed hyperreflective fluid present in HFL, whereas only 19.7% had such fluid present in the inner nuclear layer (INL)1.
In diabetic patients, the inner blood-retinal barrier (BRB) is destroyed by disruption of elements maintaining the inner BRB, including occluding of the retinal vascular endothelium, Muller cells, and astrocytes. Breakdown of the inner BRB increases serum exudation from the retinal vessels. Diabetic macular edema caused by increased exudation is a typical cause of SSPiM. The presence of SSPiM in OCTA images was associated with poor treatment response. HRM may represent subclinical extravasation of lipoproteins and/or proteins secondary to breakdown of blood-retinal barrier, and is generally observed at the border between cystoid spaces and hyperreflective fluid.
Taarnhøj et al. previously described the kinetics of lipoproteinaceous debris in the retina, suggesting that resorption of water and salt by the retinal pigment epithelium and intraretinal capillaries, accompanied by a lack of mechanism to resorb macromolecules, leads to precipitation of these macromolecules as their concentration increases2.
The resolution of SSPiM is associated with foci of HRM that can coalesce and be visualized as hard exudate clinically on funduscopic images1.
References